Innovations in CT imaging of acute stroke: Adding value, reducing dose, improving consistency
Images







Stroke remains a leading cause of mortality and permanent disability worldwide.1 Brain imaging plays a crucial role in early diagnosis and yields essential information regarding tissue integrity, a factor that is determinant in identifying patients likely to benefit from thrombolytic therapy. Readily available in most settings, computed tomography (CT) maintains a dominant role in the evaluation of patients with acute stroke. In this paper, we discuss several innovations in perfusion and CT angiography of acute stroke, which have an impact on quality, consistency, and radiation dose.
CT stroke imaging algorithm
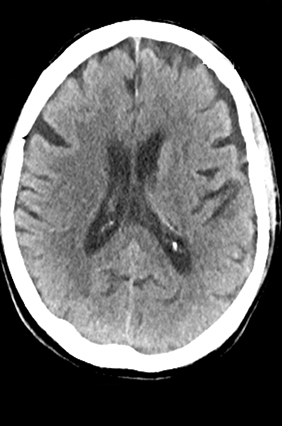
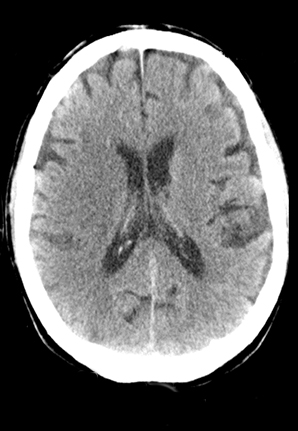
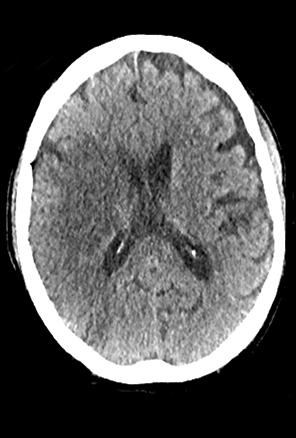
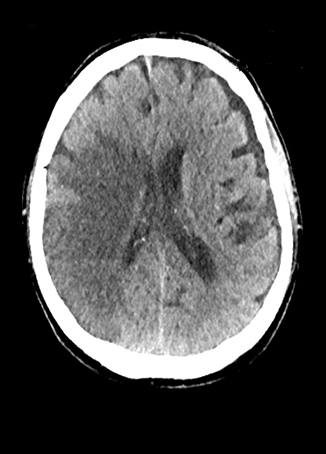
A comprehensive stroke imaging protocol commonly includes a noncontrast CT (NCCT), CT perfusion (CTP), and CT angiography (CTA) as part of the pretherapeutic diagnostic work-up. The first diagnostic test for the emergency evaluation of acute stroke, a NCCT is acquired to detect hemorrhage and the presence of infarction. Signs of acute infarction include obscuration of normal brain structures, such as the insular ribbon2 and lentiform nucleus,3 hyperdense artery,4 or in less acute cases, parenchymal hypodensity and mass effect (Figure 1). First level decisions about the appropriateness of thrombolytic therapy in patients with acute stroke are largely based on the findings from CT, as the presence of significant infarction raises the risk of hemorrhage, thus the reader must be sensitive to these often subtle changes that identify acute infarction.
When indicated because of the potential for thrombolysis, a CTP study is performed. Today’s CT scanners are capable of whole brain coverage (120-145 mm) via adaptive shuttling techniques or with an extended coverage detector (160 mm). CTP exams are conventionally performed at 80 kVp at a fixed rate of 1 to 4 sec-per-pass over a period of approximately 40 sec.5-7 Scanning begins 7 to 8 sec after the injection of a contrast bolus of 40 mL of high concentration (370-400) nonionic-iodinated contrast media administered at a rate of 4to 5 ml/sec, followed by a saline flush of 40 mL at 3 ml/sec.
Managing radiation dose
Reducing tube voltage
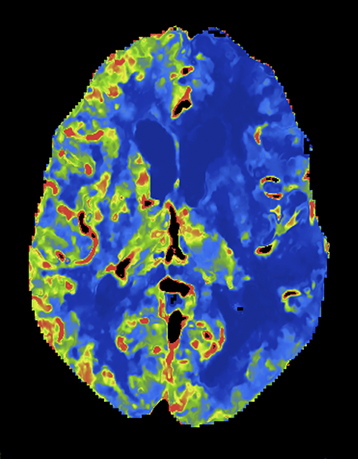
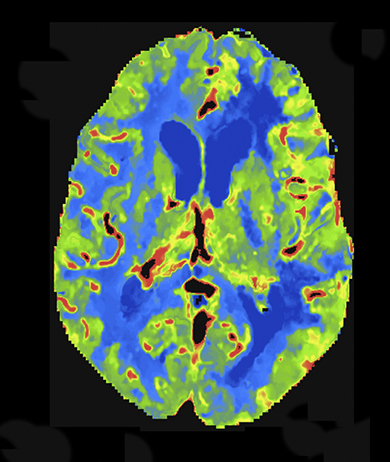
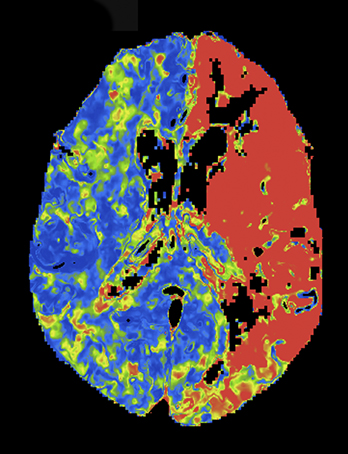
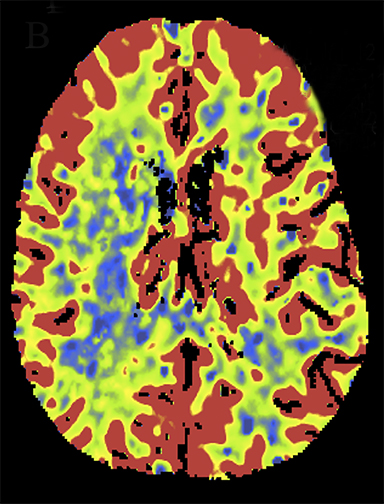
At present, the widely practiced standard for CTP is 80 kVp at 200 mAs or less.5,8 Reducing tube voltage has a disproportionate effect on dose (difference roughly squared). Wintermark et al demonstrated that moving CTP to 80 kVp from 120 kVp reduced radiation dose and made contrast enhancement more effective.5 Recent investigations have shown that reducing kilovoltage to 70 kVp offers additional advantages.9 Since 70 kVp more closely approximates the iodine k-edge (33.2 KeV),10 the effective attenuation of contrast increases further. The net result is superior CTP image quality at lower net dose per whole-brain pass. Examples are shown in Figures 2 and 3.
Adaptive variable and extended sampling
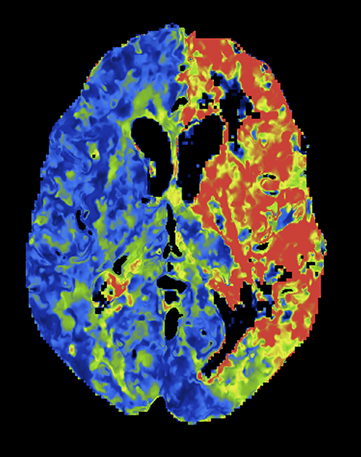
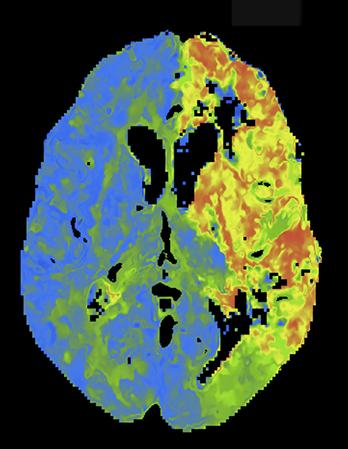
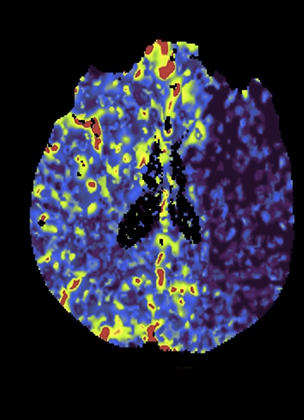
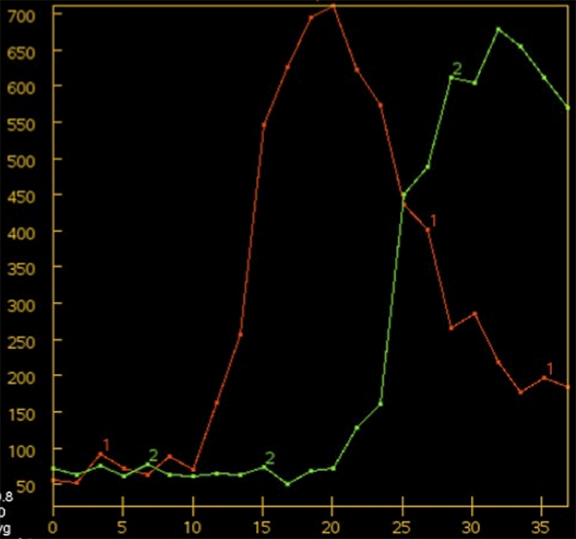
Overall scan time can be reduced in the interest of dose management. Shorter scan times increase the risk that, due to physiologic factors, such as variability in cardiac output, some exams may not continue through venous return to baseline, challenging calculation of deconvolution-based parametric maps (Figure 4).11,12 A recent retrospective study by McLellan et al showed that almost 50% of their studies performed with a ‘classic’ 40 sec protocol do not image the full contrast cycle.9 Longer sampling periods can provide greater consistency of contrast capture from arterial arrival through venous return over a broad range of cardiac outputs but require an increase in dose.9
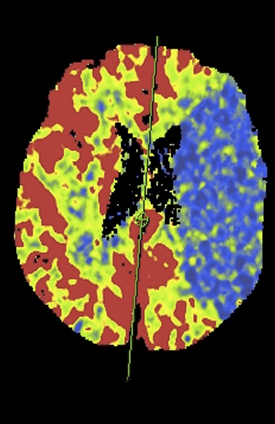
Wider CTP sampling intervals have also been employed to minimize radiation dose. While sampling rates from 1 to 4 sec have been proposed, the optimal sampling rate remains controversial6,7,12,13-16 As opposed to traditional fixed sampling approaches, which scan at a uniform rate over the entire CTP acquisition, variable sampling intervals allow strategic flexibility. As certain segments of the contrast passage are likely less critical to sample rapidly, such as the pre-enhancement baseline and the downward slope toward the return to and through baseline, making fewer passes during these segments is acceptable. The ‘omitted’ samples can be eliminated for dose reduction or traded for higher temporal resolution during the key periods of arterial tissue passage. Skipped scans can be also be traded for some at wide intervals at the end of the scanning period, affording greater protection against premature termination (Figure 5). Variable CTP sampling has been reported by investigators working with wide 320-channel detector systems with favorable results.13 Results with a conventional scanner and 4-dimensional (4D) adaptive spiral scanning (Siemens AS+ 128) were recently presented. Employing a 70-kVp protocol with variable sampling intervals, there was improved image quality at reduced dose with greater consistency of full contrast passage capture.9
Advances in stroke imaging technique
Time resolved 4D CTA
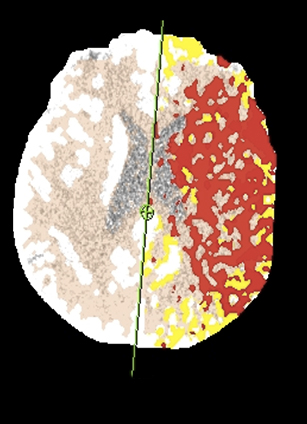
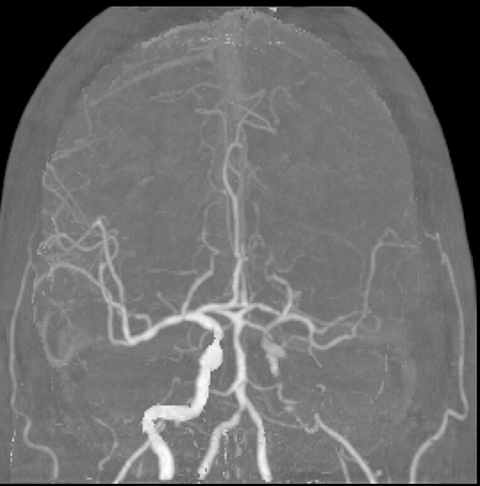
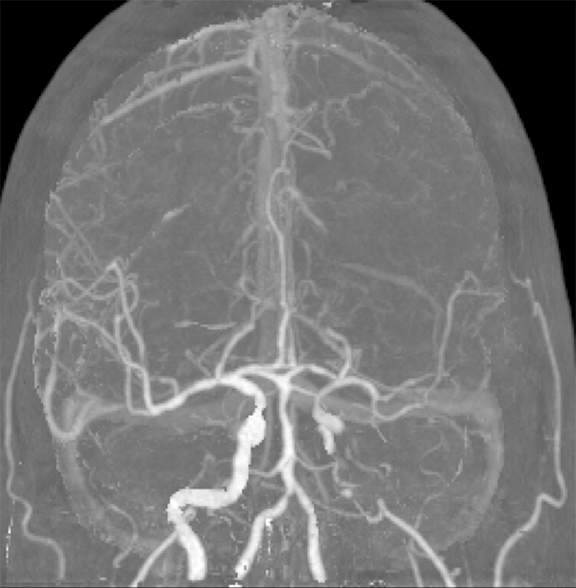
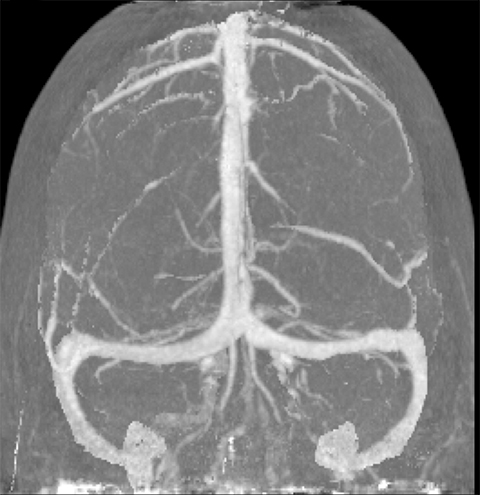
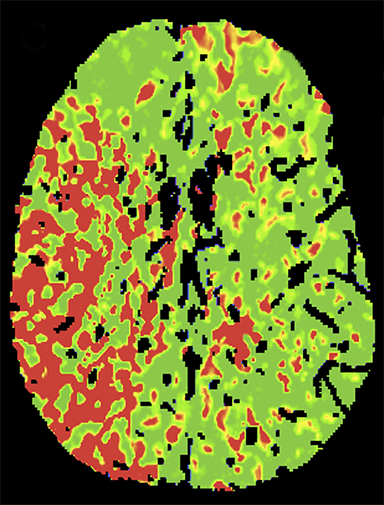
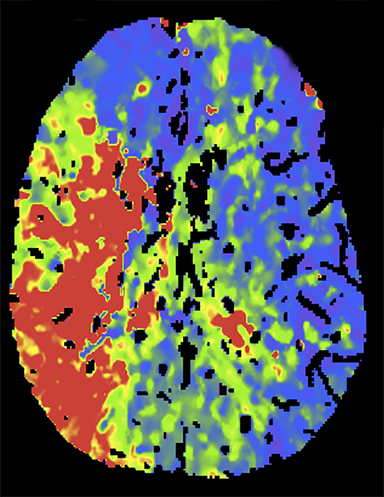
CT angiography (CTA) can depict the presence and site of stenosis and occlusion and assess collateral flow—influential factors in management decision making. Typically acquired after or before CTP at the cost of additional radiation and iodinated contrast dose, today’s whole brain evaluation techniques allow extraction of time resolved (TR) 4D CTA data directly from the CTP study. This not only makes the additional contrast and radiation dose associated with a dedicated CTA unnecessary but provides valuable dynamic vascular information, which can be useful in depicting collateral dynamics and in differentiating stenosis from occlusion (Figures 6 and 7).
Conclusion
Stroke is a leading cause of mortality and permanent disability worldwide.1 Readily available in most settings, CT remains the dominant modality in the evaluation of patients with acute stroke. CTP and CTA yield essential information regarding tissue integrity, which can be determinant in identifying patients likely to benefit from thrombolytic therapy. With appropriate translation of recent technical advances,such as 70 kVp, variable sampling, and extracted TR CTA, the CT workup can be faster, more reliable, and less x-ray dose intensive.
References
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: A report from the American Heart Association. Circulation. 2013;127:e6-e245.
- Truwit CL, Barkovich AJ, Gean-Marton A, et al. Loss of the insular ribbon: another early CT sign of acute middle cerebral artery infarction. Radiology. 1990;176:801-806.
- Tomura N, Uemura K, Inugami A, et al. Early CT finding in cerebral infarction: Obscuration of the lentiform nucleus. Radiology. 1988;168:463-467.
- Barber PA, Demchuk AM, Hudon ME, et al. Hyperdense sylvian fissure MCA “dot” sign: A CT marker of acute ischemia. Stroke. 2001;32(1):84-88.
- Wintermark M, Maeder P, Verdun FR, et al. Using 80 kVp versus 120 kVp in perfusion CT measurement of regional cerebral blood flow. AJNR Am J Neuroradiol. 2000;21:1881-1884.
- Wintermark M, Smith WS, Ko NU, et al. Dynamic perfusion CT: optimizing the temporal resolution and contrast volume for calculation of perfusion CT parameters in stroke patients. AJNR Am J Neuroradiol. 2004;25:720-729.
- Wiesmann M, Berg S, Bohner G, et al. Dose reduction in dynamic perfusion CT of the brain: Effects of the scan frequency on measurements of cerebral blood flow, cerebral blood volume, and mean transit time. Eur Radiol. 2008;18:2967-2974.
- Wintermark M, Lev MH. FDA investigates the safety of brain perfusion CT. AJNR Am J Neuroradiol. 2010;31:2-3.
- McLellan AM, Corcuera-Solano I, De Leacy R, et al. Whole brain adaptive 70 kV perfusion imaging with variable and extended sampling improves quality and consistency while reducing dose. Am S Neuroradiol. 2014; Montreal, Quebec.
- Huda W, Lieberman KA, Chang J, Roskopf ML. Patient size and x-ray technique factors in head computed tomography examinations. II. Image quality. Med Phys. 2004;31:595-601.
- Konstas AA, Goldmakher GV, Lee TY, Lev MH. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 1: Theoretic basis. AJNR Am J Neuroradiol. 2009;30: 662-668.
- Fieselmann A, Kowarschik M, Ganguly A, et al. Deconvolution-based CT and MR brain perfusion measurement: Theoretical model revisited and practical implementation details. Int J Biomed Imaging. 2011;2011:467563.
- Shankar JJ, Lum C, Sharma M. Whole-brain perfusion imaging with 320-MDCT scanner: Reducing radiation dose by increasing sampling interval. AJR Am J Roentgenol. 2010;195:1183-1186.
- Kamena A, Streitparth F, Grieser C, et al. Dynamic perfusion CT: optimizing the temporal resolution for the calculation of perfusion CT parameters in stroke patients. Eur J Radiol. 2007;64:111-118.
- Abels B, Klotz E, Tomandl BF, et al. CT perfusion in acute ischemic stroke: A comparison of 2-second and 1-second temporal resolution. AJNR Am J Neuroradiol. 2011;32:16321639.
- Kloska SP, Fischer T, Sauerland C, et al. Increasing sampling interval in cerebral perfusion CT: limitation for the maximum slope model. Acad Radiol. 2010;17:61-66.