Ovarian/adnexal masses in the nonpregnant female patient
Images















The clinical presentation and pertinent laboratory analysis often determine the appropriate imaging differential in evaluating any woman presenting with pelvic symptoms.
In a patient with a positive pregnancy test, sonography is the modality of choice to evaluate the pregnancy and any complications. In other patients who are not pregnant and present with pelvic pain or a mass, ultrasound is also the first imaging modality utilized.
Computed tomography (CT) is primarily utilized for staging of pelvic malignancies or suspected bowel abnormalities, such as appendicitis or diverticulitis. However, CT performed for other reasons, including abdominal pain, may detect and may be diagnostic in certain pelvic abnormalities.
Magnetic resonance imaging (MRI) is typically performed in situations where a pelvic mass is detected, but not fully characterized by sonography. This article emphasizes the use of ultrasound and MRI in assessing ovarian/adnexal masses. The authors will not focus on patients with a positive β-hCG or other problems, such as dysfunctional uterine bleeding or uterine enlargement.
Imaging
In evaluating any pelvic mass, it is important to first determine if the mass is arising from the ovaries, the uterus, or another location. If the anatomical location of the mass can be determined, then imaging may be extremely helpful in establishing a more precise diagnosis. For instance, if the mass is of ovarian origin, determining whether it is cystic, solid, or complex is very helpful. Likewise, identifying the presence of any fat or calcium in the mass is important.
Ultrasound
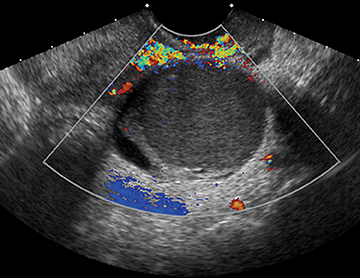
Ultrasound may be extremely helpful in evaluating cystic or solid adnexal masses.
Table 1 is a noninclusive list of common cystic and solid adnexal masses that may be detected—and in many cases diagnosed—by sonography. A comprehensive discussion of all adnexal masses is beyond the scope of this pictorial review. However, a recent article by Levine D., et al published in Radiology in 2010, summarized the Society of Radiologists in Ultrasound (SRU) consensus statement on management of asymptomatic ovarian and other adnexal cysts.1 Based on the consensus, in the premenopausal female, simple ovarian cysts that are ≤ 3 cm without color flow need not be followed. In postmenopausal females, small simple cysts ≤ 1 cm also need not be followed. This consensus statement also reviewed other common features of cysts and presented specific recommendations, which may include follow-up ultrasound at 6 to 12 weeks in certain situations. For worrisome cysts, such as those with multiple or thick septations, color flow within the septation or solid nodules, MRI or surgical evaluation is recommended. Tables 2 and 3 give a summary of the SRU recommendation. Many of these specific entities will be discussed within this article.1
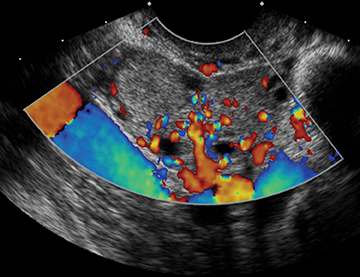
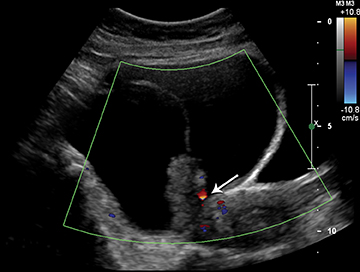
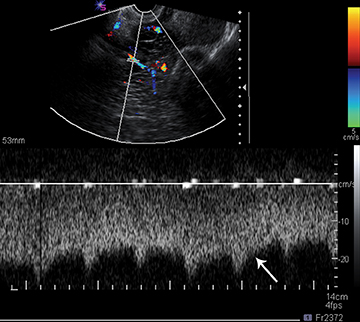
Ultrasound is very helpful in diagnosing a number of entities. Ovarian torsion is a common entity that may be diagnosed with sonography. Usually, there is an associated ovarian mass and an enlarged ovary. The torsed ovary may be in an abnormal position, with or without decreased color flow. At times, the ovaries may “torse” then “detorse.” Thus classically there is decreased color and Doppler flow to the ovary, but with detorsion, this pattern is reversed. There may also be free fluid in the pelvis and/or abdomen associated with ovarian torsion.
Tubo-ovarian or other pelvic abscesses occur from a number of etiologies in the female patient. Usually there is an appropriate history of fever, elevated white blood cell count, and adnexal tenderness, which will indicate such a process. Hydrosalpinx or pyosalpinx often accompany these cases. Patients with a perforated appendix or other gastrointestinal perforations may present with a secondary pelvic abscess. These will usually be localized in the cul-de-sac and are most commonly diagnosed with CT and/or ultrasound. MRI has a limited role in these cases.
Several ovarian and/or adnexal masses have specific ultrasound features. For instance, in the correct clinical setting, classic findings of multiple small peripheral follicles within a slightly enlarged ovary may represent polycystic ovarian disease. Likewise, in ovarian hyperstimulation, whether due to drug stimulation for infertility or gestational trophoblastic disease, bilateral enlarged ovaries have multiple cysts called theco-luteal cysts. Some other entities presented in Table 1 will not be presented in detail in this review.
Computed tomography
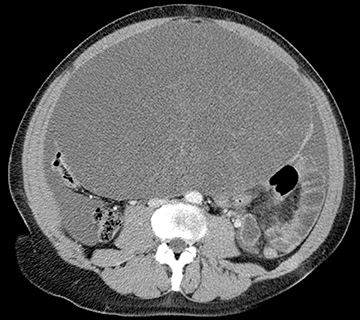
CT may be helpful for staging and evaluating the extent of malignancy. CT may be used to diagnose appendicitis or diverticulitis. In other circumstances, CT may also be helpful to determine the location of the mass and if it is cystic, solid, or complex. CT is helpful to identify fat, calcium, or air within a structure. Thus, in some cases, such as a dermoid cyst, CT findings are fairly specific.
Magnetic resonance imaging
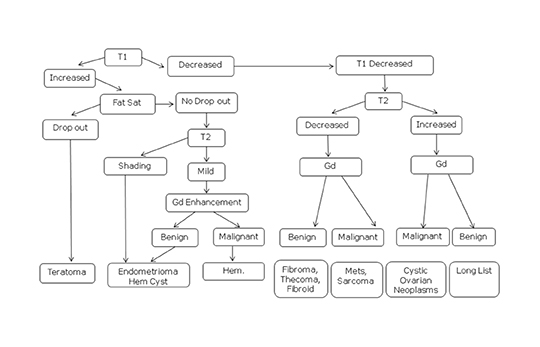
Pelvic MRI is helpful in difficult cases and may provide a more definitive diagnosis. MRI is commonly utilized for evaluation of cystic or solid adnexal or ovarian masses in which ultrasound cannot be diagnostic or when there are worrisome or indeterminate ultrasound features. MRI may identify the location of the mass and determine if it is a cystic, solid, or complex. MRI may be useful in identifying macroscopic fat within a mass and thus can be diagnostic of a dermoid. A mass that contains hyperintense foci on T1-weighted imaging loses signal after application of fat saturation contains macroscopic fat. Old hemorrhage can be identified within a mass as “T2 shading” with MRI and suggests of an endometrioma. T2 shading refers to a lesion which is bright on a T1-weighted sequence, but which decreases in signal intensity on a T2-weighted sequence.2 MRI may show solid ovarian masses with decreased signal intensity on both T1 and T2 images, which may indicate a fibroma, fibromathecoma, or Brenner’s tumor. Exophytic uterine fibroids can also have these imaging characteristics and can mimic ovarian masses. Finally, more aggressive ovarian neoplasms will have a combination of findings on MRI suggestive of an ovarian malignancy.
Case example
Germ cell tumors
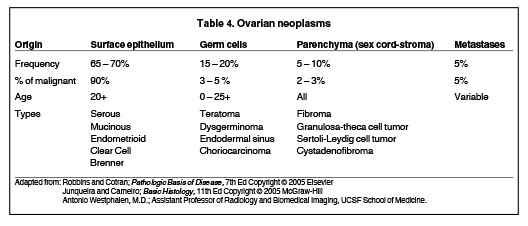
Dermoid cysts and teratomas are classified as germ cell tumors (Table 4). They account for 15% to 20% of all ovarian neoplasms and are rarely malignant. Teratomas may be mature or immature. Dermoid cysts are mature cystic teratomas that may have components from 3 germ-cell layers that predominantly include mature ectoderm elements.
Ultrasound/CT features
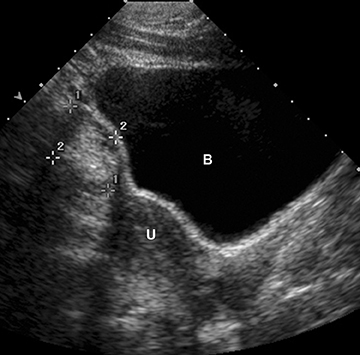
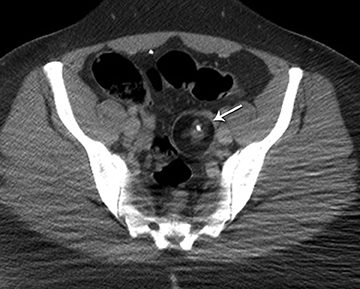
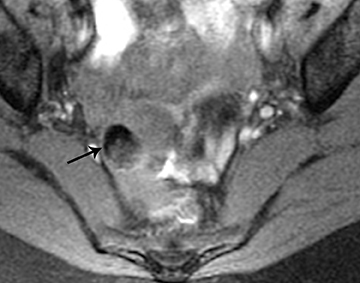
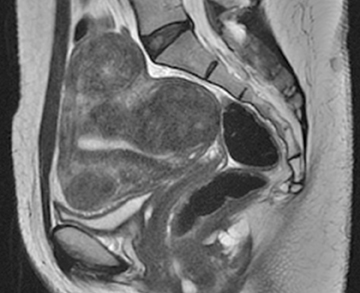
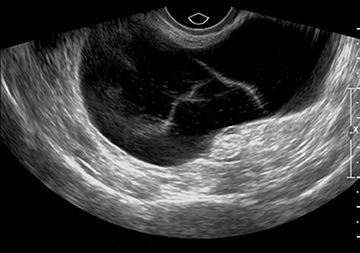
Ultrasound and CT features of dermoids include demonstration of cystic components, fatty elements, and/or calcifications (Figure 1). Thus, on ultrasound, an echogenic shadowing focus in a predominantly cystic mass is highly predictive of a dermoid cyst. However, there may be a variety of other sonographic features of a dermoid cyst. These can include a hyperechoic line and dots or an echogenic mass in which the distal portion is not seen as with a “tip of an iceberg.” They may appear as complex masses with a dermoid plug (a Rokitansky nodule). This nodule is a solid mass of sebaceous material projecting into the lumen of the mass. These features are present in Figure 2.
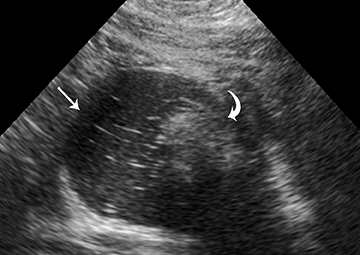
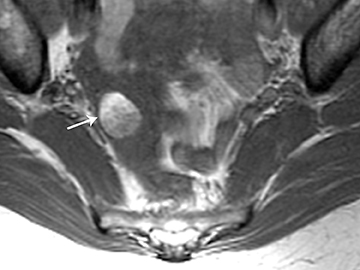
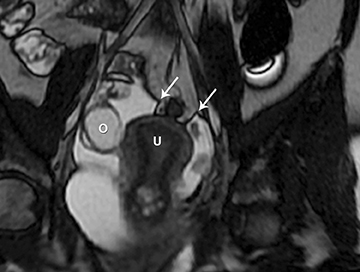
MRI may also be very helpful in distinguishing teratomas from other adnexal masses by demonstrating macroscopic fat within the ovarian mass (Table 5). Thus on T1-weighted images, the mass is hyperintense, which could reflect fat, hemorrhage, or other proteinaceous materials. However, signal loss after applying fat saturation indicates macroscopic fat and is characteristic of a teratoma (Figure 3). Most teratomas are mature and are also called dermoids. Immature teratomas are very rare and constitute only 1% of all teratomas. They grow rapidly, are seen in younger patients and are often malignant. Dysgerminoma is another unusual germ cell tumor and is usually a multilobulated solid mass, often having a low-attenuation center on CT. Dysgerminomas occur in young women and do not have the typical appearance of a mature teratoma (Figure 4).
Stromal tumors (sex cord)
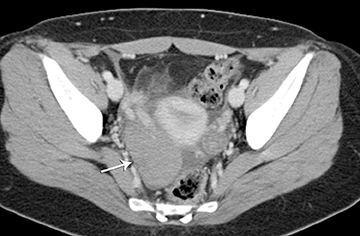
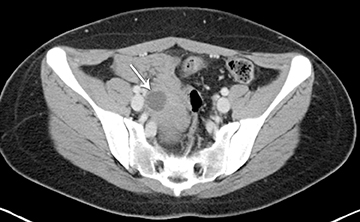
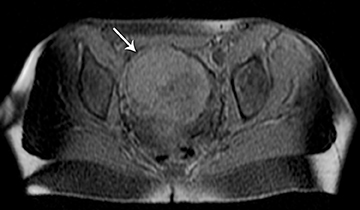
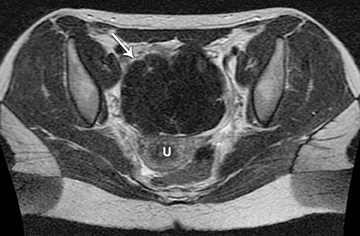
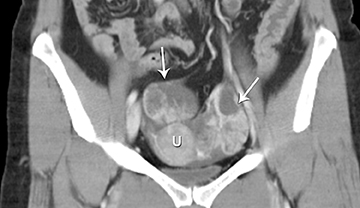
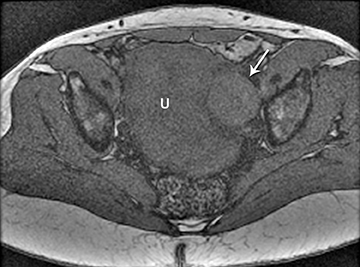
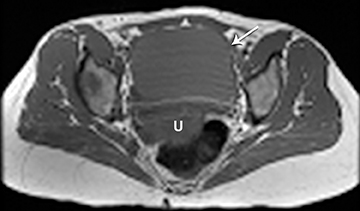
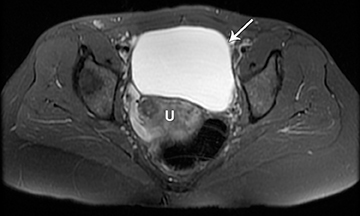
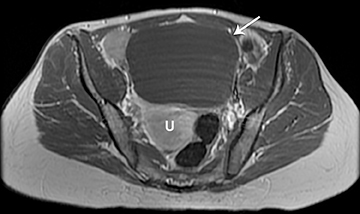
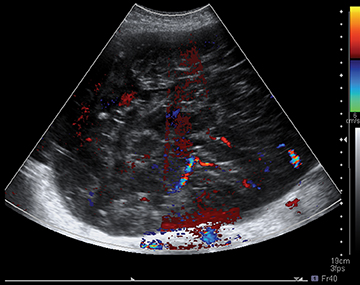
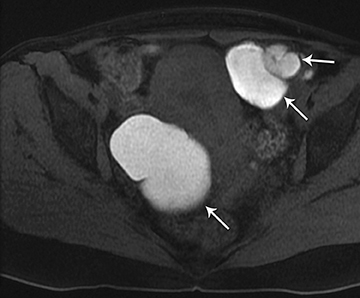
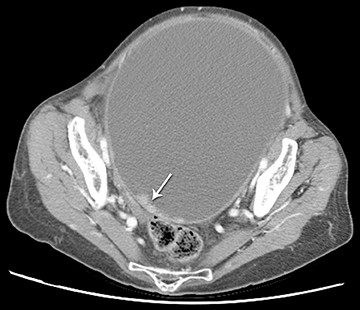
This is an interesting group of tumors that appear as solid ovarian masses (Table 4). Sex cord tumors are rarely malignant. This group is composed of a variety of tumors including fibroma fibrothecoma, cystadenofibroma, Sertoli- and Leydig cell tumors. More common sex-cord stromal tumors include components of fibrous tissue, which are fibromas and/or lipid-rich tissues, which include thecomas. Many of these tumors have combined elements, such as a fibrothecoma. These are the most common solid ovarian neoplasms seen in postmenopausal females. Rarely, they may result in Meig’s syndrome (fibroma with ascites and pleural effusions). Ultrasound can determine the solid nature of these neoplasms (Figure 5). MRI will demonstrate a solid tumor with decreased signal intensity on T1 and T2 images (Table 5, Figure 6).3 Thus, the differential diagnosis includes a solid ovarian mass such as fibroma, fibrothecoma, or cystadenofibroma. Potential pitfalls include a Brenner’s tumor, which is usually solid and the presence of cysts as a component of these predominantly solid tumors that can be hypotintense on T2-weighted imaging. Also, ovarian metastases, including Krukenberg tumors, may be mistaken as a solid sex-cord stromal tumor and can appear as solid or complex ovarian masses (Figure 7).4 However, most solid ovarian masses will probably be surgically removed except in the case of a pedunculated fibroid that can mimic an ovarian mass (Figure 8). It may be difficult to distinguish a benign solid ovary mass from a solid metastasis.
Surface epithelia tumors
The most worrisome tumors detected or evaluated with ultrasound, CT, or MRI are surface epithelial neoplasms (Table 4). These can include serous or mucinous cystadenomas and serous or mucinous cystandenocarcinomas. Serous neoplasms have a higher frequency of malignancy than mucinous ovarian neoplasms (Figure 9). Clear cell carcinomas are another type of surface epithelial neoplasm that is predominantly cystic with few solid components (Figure 9). Clear cell carcinomas account for 5% of all ovarian cancers. They are predominantly cystic, much like endometriomas, but do have solid components along the wall of the tumor. Obviously, surface nodularity may be indicative of a malignant neoplasm, which would thus alter management (Figure 9).
Mucinous tumors often have multiple septations with the appearance of multiple cysts. Serous tumors may have a completely benign appearance when they are cystadenomas (Figure 10). Worrisome features include septations with color flow, and papillary projections within the cystic portions of the tumor. It is important to identify imaging features on ultrasound or MRI that may differentiate benign from malignant cystic ovarian neoplasms. Usually malignant neoplasms have a heterogeneous architecture, are larger, and have fairly thick walls. Septations are often thick and/or irregular with regions of enhancement. Mural nodularity and necrosis are often present on ultrasound or MRI (Table 6; Figures 11 and 12). At times, some of these tumors may appear malignant on ultrasound and/or MRI, but are considered pathologically as borderline tumors (Figure 13). These borderline tumors have a low malignant potential; they occur in the younger population with a fairly good 10-year survival rate, as high as 95%.5 Ovarian metastases are not surface epithelial neoplasms, but may mimic some of these tumors and may be more solid or mixed than cystic.
Other Cystic/Complex Adnexal Masses
Peritoneal Inclusion Cysts
One mimic of a surface epithelial neoplasm is a benign entity called a peritoneal inclusion cyst, which may be mistaken for a complex cystic ovarian mass. Peritoneal inclusion cysts represent fluid “trapped” within the peritoneal adhesions in patients with previous history of abdominal or pelvic surgery, Crohn’s disease, or any other prior pelvic inflammatory process. These “cysts” are not spherical, but they may be oblong with more acute angulations at their margin, as the fluid is interposed between different surfaces within the pelvis (Figure 14).6 A history of prior surgery is helpful in such patients. There is no evidence of mural nodularity or enhancement on MRI.
Endometriomas
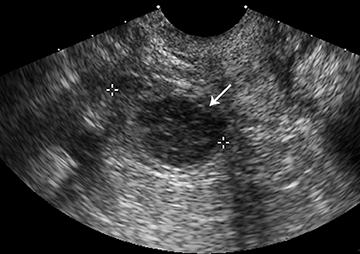
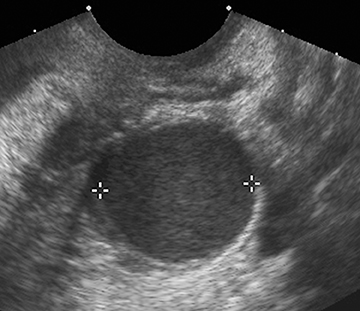
Endometriomas and hemorrhagic cysts may appear similar on sonography. However, a hemorrhagic cyst may have a characteristic “fish net” appearance with a reticular pattern of internal echoes (Figure 15). According to the SRU consensus, if a mass with this appearance is identified and is < 5 cm without internal flow, then no follow-up is needed. However if the mass is > 5 cm, follow-up at 6 to 12 weeks is recommended. Endometriomas usually appear with homogeneous low-level internal echoes with no solid components on ultrasound (Figure 16). Follow-up at 6 to 12 weeks is recommended in these cases.
On MRI, endometriomas most commonly have increased signal intensity on T1-weighted images and no loss of signal on fat saturation images. However, on T2-weighted images there is decreased signal intensity of the mass which has been termed “T2 shading.”2 This is a fairly classic version of endometriomas, but can also be identified with hemorrhagic cysts or with hemorrhage occurring with certain neoplasms (Figure 17). On MRI, gadolinium enhancement may help to identify foci of endometrial implants elsewhere within the pelvis. Gadolinium also may help to ensure there are no regions of enhancement along the wall of the presumed endometrioma, as this may be indicative of a necrotic ovarian tumor.
Conclusion
Both ultrasound and CT play a significant role in diagnosing ovarian and adnexal pathology. Ultrasound is usually the screening modality of choice and may be useful in establishing a specific diagnosis in the nonpregnant patient. However, at times, MRI with gadolinium is needed to establish a more specific diagnosis and may be used to problem solve in certain cases.
REFERENCES
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256:943-954.
- Woodward PJ, Sohaey R, Mezzetti TP Jr. Endometriosis: Radiologic-pathologic correlation. Radiographics. 2001;21:193-216.
- Jung SE, Lee JM, Rha SE, et al. CT and MR imaging of ovarian tumors with emphasis on differential diagnosis. Radiographics. 2002;22:1305-1325.
- Saini A, Dina R, McIndoe GA, et al. Characterization of adnexal masses with MRI. AJR Am J Roentgenol. 2005;184:1004-1009.
- DeSouza NM, O’Neill R, McIndoe GA, et al. Borderline tumors of the ovary: CT and MRI features and tumor markers in differentiation from stage I disease. AJR Am J Roentgenol. 2005;184:999-1003.
- Jain KA. Imaging of peritoneal inclusion cysts. AJR Am J Roentgenol. 2000;174:1559-1563.
Citation
. Ovarian/adnexal masses in the nonpregnant female patient. Appl Radiol.
May 1, 2014