Artificial Intelligence-Assisted Peer Review in Radiation Oncology
Objective
Peer review is an essential part of the patient treatment process that examines and, where necessary, recommends revisions to clinical data, therapeutic parameters, and potential alternative approaches to treatment. Our hypothesis is that artificial intelligence (AI) and machine language technologies can enhance peer-review efficacy by screening cases for potential treatment interruptions caused by re-planning and treatment cessation.
Materials and Methods
Fifty-five features of clinical and therapeutic parameters from 3881 radiotherapy patients (7142 plans) treated from 2014 to 2021 were used as input for two AI models: a multivariable least absolute shrinkage and selection operator (LASSO) logistic regression model and a pattern recognition feed-forward neural network (NN). The dataset was split into 70% training and 30% testing, with the training set divided into five groups for cross-validation. Analysis was performed on the full cohort and on subsets based on treatment site. Performance metrics of accuracy, sensitivity, and specificity were calculated.
Results
Overall, 8.1% of all cases had treatment interruptions, most commonly in the head and neck region compared to other sites (19% vs 6%-9%, P <.01). For the LASSO model, test set sensitivity, specificity, and accuracy ranged from 37%-70%, 59%-78%, and 60%-76%, respectively, with higher specificity than sensitivity for site subsets. For the NN model, test set sensitivity, specificity, and accuracy ranged from 41%-68%, 53%-79%, and 53%-78%, respectively. Both models demonstrated the highest accuracy in the brain subset. For the full cohort, NN accuracy (58%) was similar to LASSO (60%). The largest accuracy differences between LASSO and NN were in the lung/breast/chest (LASSO: 71% vs NN: 57%) and spine/extremity (LASSO: 66% vs NN: 54%) subsets.
Conclusion
Our results provide proof-of-concept that AI- and ML-based technologies have potential as screening tools to aid peer review in radiation oncology. Early identification of patients at risk for radiation therapy interruptions using these tools could translate into higher treatment completion rates. The study is being continued to include more clinical features and to optimize model hyperparameters.
Keywords: peer review, artificial intelligence, machine learning, radiation oncology
Introduction
Peer review in radiation oncology is essential for the safe and efficient delivery of radiation treatments;1 however, little guidance and limited research exist regarding the frequency, mechanisms, and metrics of peer review from professional organizations.1 - 4 Peer review addresses patient characteristics and clinical oncological information, planned radiotherapeutic parameters, and potential treatment-related variations. In a report series commissioned by the American Society for Radiation Oncology, seven main items are currently examined by peer reviewers.1 These are (1) the decision to include radiation as part of treatment, (2) the general radiation treatment approach, (3) the target definition, (4) normal tissue image segmentation, (5) the planning directive, (6) technical plan quality, and (7) treatment delivery.
Many of these factors are indicative of cases requiring re-plans or cases that may have treatment interruptions. Although peer reviews should be conducted prior to the start of treatment to ensure safety and quality, they are typically performed either immediately before or after treatment has begun. Thus, there is minimal time to adjust therapy based on the recommendations of peer reviewers. Plan changes based on peer review discussions are not uncommon. Hoopes et al reported that 90% of physicians have changed their radiation plans because of peer review.3 Other studies have shown that up to 10% of cases have been recommended for plan modifications based on peer review.1, 3 Studies have also demonstrated that prolonged radiation treatment times, for various reasons, correlated with reduced local disease control and worse overall survival.5 - 7 Therefore, timely, efficient peer review may greatly improve treatment quality and, ultimately, enhance patient safety.
However, peer review has some practical difficulties, especially when the process must be coordinated among multiple facilities. While a fully integrated approach holds promise for improving the quality, safety, and value of cancer care, in reality, the process is often disjointed owing to differing provider schedules, caseloads, and expertise. These opportunities to optimize peer review are further highlighted by the fact that different approaches are taken by academic centers and community cancer centers operating within the same network.8 Practically speaking, however, caseload, time, department resources, staffing, and increasing complexity in treatment techniques limit thorough peer review.9 Thus, detecting cases that may experience treatment interruptions due to undesirable effects is not easy.
A pan-Canadian survey by Caissie et al demonstrated that barriers to peer review, including time constraints (27%) and radiation oncologist availability (34%), caused half of all programs surveyed to conduct peer review after the start of treatment.10 Therefore, automating peer review, even if only partially, may significantly streamline the process.
Artificial intelligence (AI) tools can potentially be used to assist in peer review. Studies have used machine learning (ML) techniques to identify high-risk patients for detailed clinical evaluation during radiation and chemoradiation.11 To identify potential treatment interruptions or unusual side effects resulting from suboptimal treatment plans, we hypothesize that complex, nonlinear relationships exist between different variables, including clinical characteristics and plan parameters. Our approach was to use AI and ML to uncover complex interactions among variables and to supplement clinical knowledge with treatment and plan parameters. By using AI, our intent was to screen treatment plans for clinical and radiotherapeutic factors that could lead to treatment interruptions or toxicity and submit the plans for more detailed inspection to help expedite peer review.
Methods and Materials
Data Collection
This retrospective study was approved by the Institutional Review Board. Clinical data and radiation therapy plan parameters from 3881 patient records (7142 plans) were retrospectively extracted from electronic medical records and treatment planning systems from January 2014 to March 2021 for patients older than 18 years. The study inclusion criteria consisted of patients who received external beam photon radiation therapy with either curative or palliative intent for initial treatment and/or re-treatment. This study excluded patients who underwent brachytherapy and electron therapy. Included patients were further divided into subsets based on bodily treatment sites ( Table 1 ). Comparisons were made using two-tailed t tests with unequal variance.
Distribution of Plans With and Without Treatment Interruptions Across Separate Subset Groups. “All” Indicates the Full Cohort Before Separated into Subsets Based on Treatment Site
| Treatment Site | Total Number of Plans | Plans With Treatment Interruptions | Plans Without Treatment Interruptions |
|---|---|---|---|
| All | 7142 | 579 (8.1%) | 6563 (91.9%) |
| Lung, breast, and chest | 3329 | 228 (6.8%) | 3101 (93.2%) |
| Pelvis and prostate | 1077 | 90 (8.4%) | 987 (91.6%) |
| Spine and extremity | 983 | 92 (9.4%) | 891 (90.6%) |
| Brain | 1285 | 81 (6.3%) | 1204 (93.7%) |
| Head and neck | 468 | 88 (18.8%) | 380 (81.2%) |
The aim of this study was to identify patients likely to experience treatment interruptions (eg, changes in target volume, changes in prescription dose) or complications (eg, toxicity). As a surrogate for this outcome, plans with treatment interruptions (remaining fractions) were designated as abnormal cases, whereas radiation therapy plans with no remaining fractions (ie, no interruptions) were considered to be normal cases. We defined remaining fractions as any plan that was not completed; we did not separate re-planned cases versus discontinued cases.
Most interruptions are the result of toxicity or treatment response based on radiosensitivity and tumor histology. This surrogate, although imperfect, was easily translated into an instance that could be extracted automatically from the patient records. We note that the specific reason(s) for treatment interruption were not included in this study, as our intention was to identify potential cases and prevent treatment modification. Various clinical factors and therapeutic technical parameters were collected for logistic regression analysis and developing neural network (NN) models. The input factors are listed in Table 2 .
Input Features for the LASSO and NN Models
| Clinical Features | Plan Parameter Features |
|---|---|
| Patient sex (male, female) | Number of beams |
| Patient age group in years ( ≥75, 50-74,19-49) | Type of plan (IMRT, RapidArc, other) |
| Site | Monitor units (total, average, minimum, maximum) |
| Primary or metastasis | Source to skin distance (SSD; average, minimum, maximum) |
| Inpatient status | Table tolerance |
| Personal history of cancer | Gantry angle (minimum, maximum) |
| Family history of cancer | Bolus |
| Tobacco use | Gating |
| Smoker | Collimator angle (average, minimum, maximum) |
| Second hand smoke | Couch lateral (maximum) |
| Obesity | Couch longitudinal (maximum) |
| Alcohol | Couch vertical (maximum) |
| Human papillomavirus (HPV) | Couch angle (average, minimum, maximum) |
| Immunosuppression | Field size (average, minimum, maximum) |
| Immunodeficiency | Isocenter ( X , Y , Z ) |
| Neutropenia | Maximum beam energy |
| Anemia | Dose per fraction |
| Human immunodeficiency virus (HIV) | |
| Hepatitis | |
| Heart disease | |
| Diabetes | |
| Hypertension | |
| Hyperlipidemia | |
| Failure to thrive |
Abbreviations: LASSO, least absolute shrinkage and selection operator; NN, neural network; IMRT, intensity-modulated radiation therapy.
Predictive Modeling
The two classification models used in this study were a multivariable logistic regression with least absolute shrinkage and selection operator (LASSO) and a pattern recognition feed forward NN. For LASSO, the maximum number of non-zero coefficients was set to 10. The NN model had one hidden layer with 10 neurons, and the weights were initialized with random seeds. The scaled conjugate gradient algorithm was used for training, and the mean absolute error was the cost function. Regularization was performed using error weights to prevent overfitting of the NN model due to the unbalanced dataset; entry samples with treatment interruptions had two times the weight of those without treatment interruption for training.
For both techniques, the dataset was first split into a 70% training set and a 30% testing set. The training set was normalized with z-score normalization, with the same center and standard deviation normalization applied to the testing set. The training set was further divided into five folds for cross-validation. Within each cross-validation, the minority class was oversampled using adaptive synthetic sampling.12 The validation fold was not oversampled. The testing set was not oversampled or included in the training of the algorithms. The number of input features to each model was 55. MATLAB (2022a, The MathWorks, Inc., Natick Massachusetts, United States) was used for development, training, and evaluation of both models.
Predictive Performance Evaluation
The full cohort and subsets were analyzed based on treatment site. The optimal operating point threshold of the receiver-operating characteristic curve was determined only from the training set. This same threshold was applied to the validation and testing sets. Sensitivity, specificity, and accuracy were calculated. The model with the greatest validation accuracy across the five-fold cross-validation was used to predict the independent testing set to assess predictive performance. Comparisons were made using two-tailed paired t tests.
RESULTS
Description of Cohort
A total of 3881 patients with 7142 plans made up the full cohort. Of the cohort, 58.1% were between 50 and 74 years, 33.1% were >75 years, and 8.8% were between 19 and 49 years. There were more females than males (59.6% vs 40.4%). The most common treatment sites were the breasts (30.2%), brain (18.0%), and lungs (13.0%). Most patients were treated with primary definitive intent (74.6%) versus treatment of metastatic disease (25.4%). Table 1 summarizes the study cohort. As shown, 8.1% of all plans experienced treatment interruptions. The head-and-neck subset had the largest percentage of treatment interruptions (18.8%), while the other subsets ranged from 6.3% to 9.4% ( P < .01).
Testing Set Breakdown
The model with the highest accuracy from the five-fold cross-validation was selected as the predictive model on an independent testing set. The breakdown of plans with and without treatment interruptions in the selected folds are shown in Table 3 . If the same fold was selected for the LASSO and NN models, the split was the same for training and validation sets. If a different fold was selected, the number of positive samples may be slightly different, owing to randomization during the splitting process. For the testing set, the split was the same for LASSO and NN because this was a hold-out set and not involved in the training process.
Distribution of Plans With and Without Treatment Interruptions Across Separate Subset Groups from the Selected Cross-Validation Fold With the Highest Validation Set Sensitivity. Numbers Shown Indicate the Number of Plans With Treatment Interruptions Divided by the Total Number of Plans. “All” Indicates the Full Cohort Before Separated into Subsets Based on Treatment Site
| Training Set | Validation Set | Testing Set | ||||
|---|---|---|---|---|---|---|
| LASSO | NN | LASSO | NN | LASSO | NN | |
| All | 315/3999 (7.9%) | 297/3999 (7.4%) | 76/1001 (7.6%) | 94/1001 (9.4%) | 188/2142 (8.8%) | 188/2142 (8.8%) |
| Lung, breast, and chest | 129/1864 (6.9%) | 141/1864 (7.6%) | 42/467 (9.0%) | 30/467 (6.4%) | 57/998 (5.7%) | 57/998 (5.7%) |
| Pelvis and prostate | 50/603 (8.3%) | 51/604 (8.4%) | 13/152 (8.6%) | 12/151 (7.9%) | 27/322 (8.4%) | 27/322 (8.4%) |
| Spine and extremity | 56/550 (10.2%) | 56/550 (10.2%) | 11/139 (7.9%) | 11/139 (7.9%) | 25/294 (8.5%) | 25/294 (8.5%) |
| Brain | 32/719 (4.5%) | 32/719 (4.5%) | 13/181 (7.2%) | 13/181 (7.2%) | 36/385 (9.4%) | 36/385 (9.4%) |
| Head and neck | 47/262 (17.9%) | 50/262 (19.1%) | 14/67 (20.9%) | 11/67 (16.4%) | 27/139 (19.4%) | 27/139 (19.4%) |
Abbreviations: LASSO, least absolute shrinkage and selection operator; NN, neural network
LASSO Performance
The performance metrics for the LASSO model are shown in Figure 1 and Supplemental Table A ( www.appliedradiationoncology.com ). For the training set, the average sensitivity, specificity, and accuracy ranged from 69% to 92%, 52% to 67%, and 67% to 79%, respectively. Sensitivity was significantly higher than specificity for the full cohort, the spine/extremity subset, and the brain subset ( P <.05). For the validation set, the average sensitivity, specificity, and accuracy ranged from 42% to 82%, 49% to 64%, and 51% to 64%, respectively. Sensitivity remained significantly higher than specificity only for the full cohort ( P <.05).
Predictive performance of the least absolute shrinkage and selection operator (LASSO) model for (A) training, (B) validation, and (C) testing datasets. For the training and validation datasets, it is the average across five-fold cross-validation. “All” indicates the full cohort before separation into subsets based on treatment site and error bars indicate standard deviation.
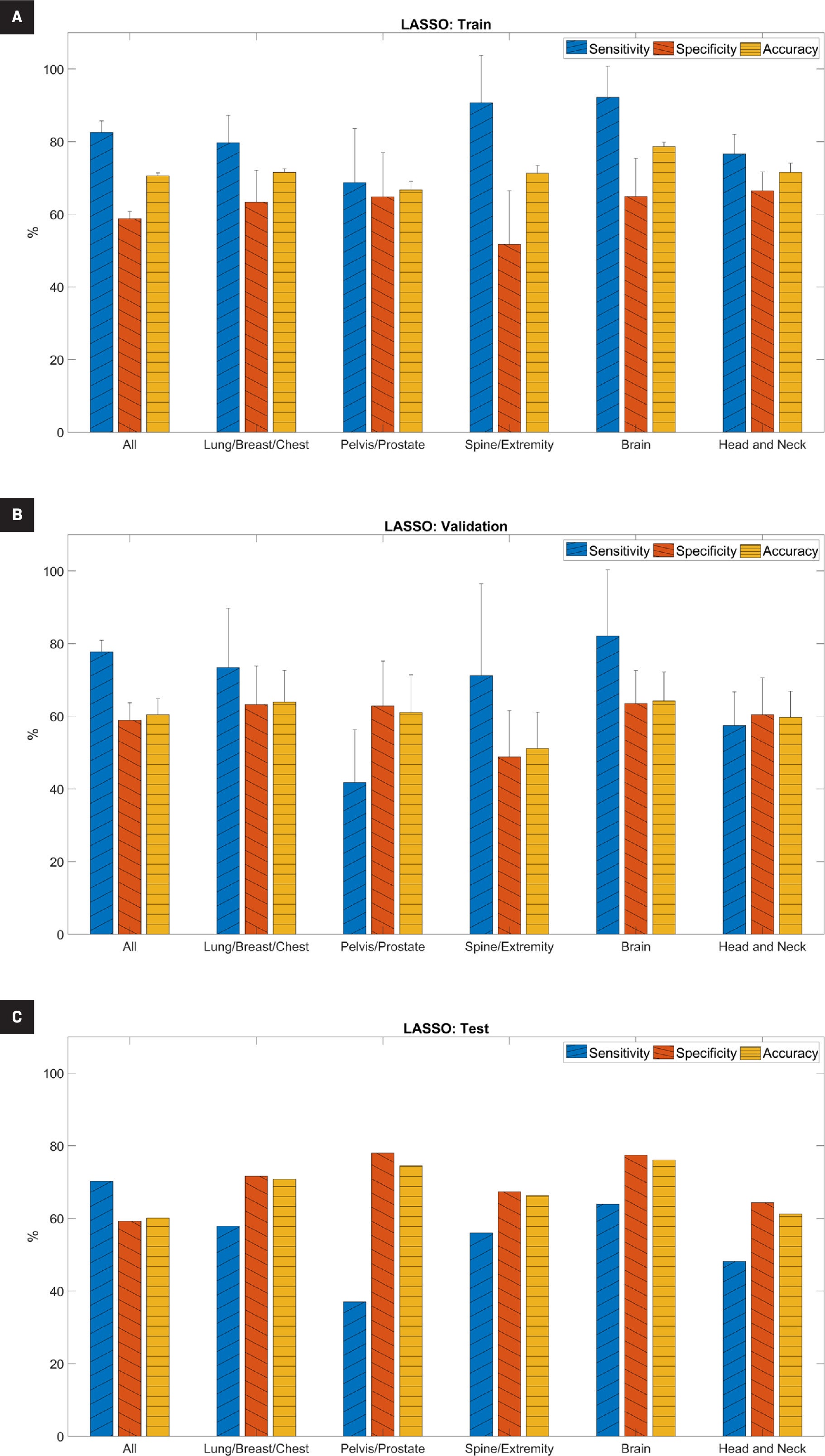
For the independent testing set, the sensitivity, specificity, and accuracy ranged from 37% to 70%, 59% to 78%, and 60% to 76%, respectively. Except for the full cohort, specificity was higher than sensitivity. The brain subset had the highest accuracy (76.1%). Overall, the higher specificity than sensitivity on the independent testing set indicated that the model was better able to predict true negatives (those without treatment interruptions) than true positives (those with treatment interruptions).
For the LASSO model, Table 4 shows the features selected by the algorithm for the prediction, which included the components of clinical features and plan parameters.
Features Selected by Logistic Regression Least Absolute Shrinkage and Selection Operator (LASSO) Model for Each Subset Group. “All” Indicates the Full Cohort Before Separation into Subsets Based on Treatment Site. Isocenter Location X , Y , and Z Refer to Lateral, Anterior/Posterior, and Superior/Inferior Directions, Respectively
| Site | Clinical Features | Plan Features |
|---|---|---|
| All |
|
|
| Lung, breast and chest |
|
|
| Pelvis and prostate |
|
|
| Spine and extremity |
|
|
| Brain |
|
|
| Head and neck |
|
|
Neural Network Performance
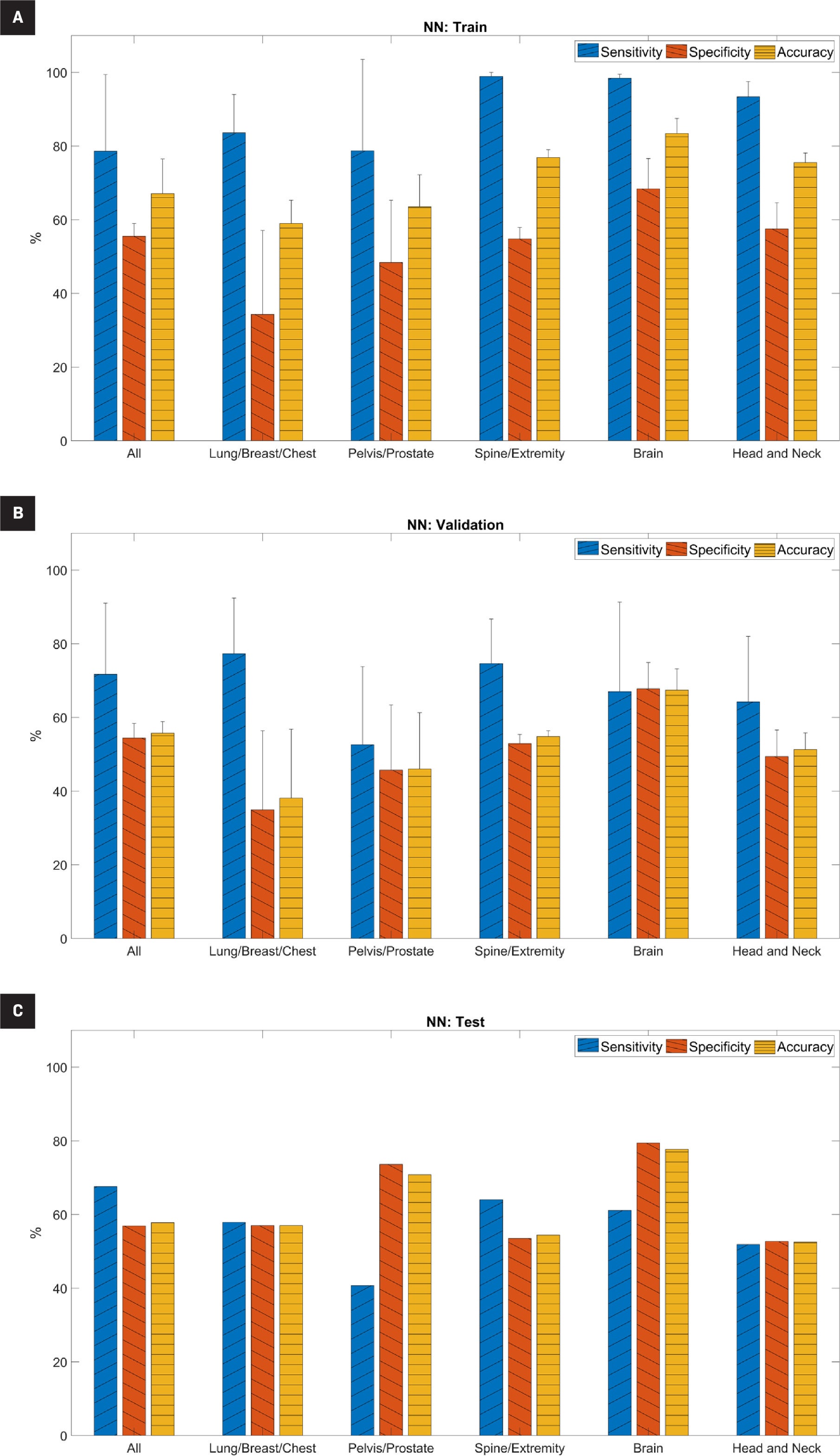
The performance metrics for the NN model are shown in Figure 2 and Supplemental Table B ( www.appliedradiationoncology.com ). For the training set, the average sensitivity, specificity, and accuracy ranged from 79% to 99%, 34% to 68%, and 59% to 83%, respectively. Sensitivity was significantly higher than specificity ( P <.05) for all subsets except the pelvis/prostate subset ( P =.15). For the validation set, average sensitivity, specificity, and accuracy ranged from 53% to 77%, 35% to 68%, and 38% to 67%, respectively. We observed a similar trend in the validation set as in the training set; sensitivity in the validation set was generally higher than specificity, although it was only statistically significant in the spine/extremity subset ( P <.05). For the testing set, the sensitivity, specificity, and accuracy ranged from 41% to 68%, 53% to 79%, and 53% to 78%, respectively. Overall, accuracy was highest in the brain subset (78%).
Predictive performance of the neural network (NN) model for (A) training set, (B) validation set, and (C) testing set. For the training and validation sets, it is the average across five-fold cross-validation. “All” indicates the full cohort before separation into subsets based on treatment site and error bars indicate standard deviation.
DISCUSSION
These study results provide proof of concept that AI can be a reliable screening tool in the peer review process to help identify cases early on that may cause treatment interruption or major changes in treatment course. We found that logistic regression and NN-based models had some predictive power in recognizing cases that would experience treatment interruptions. This could be useful in identifying cases that will require a replan for various reasons, such as a patient who experiences toxicity or has a dramatic change in target volume. Cases identified as “high risk” for interruptions could be highlighted in peer review for a more in-depth evaluation.
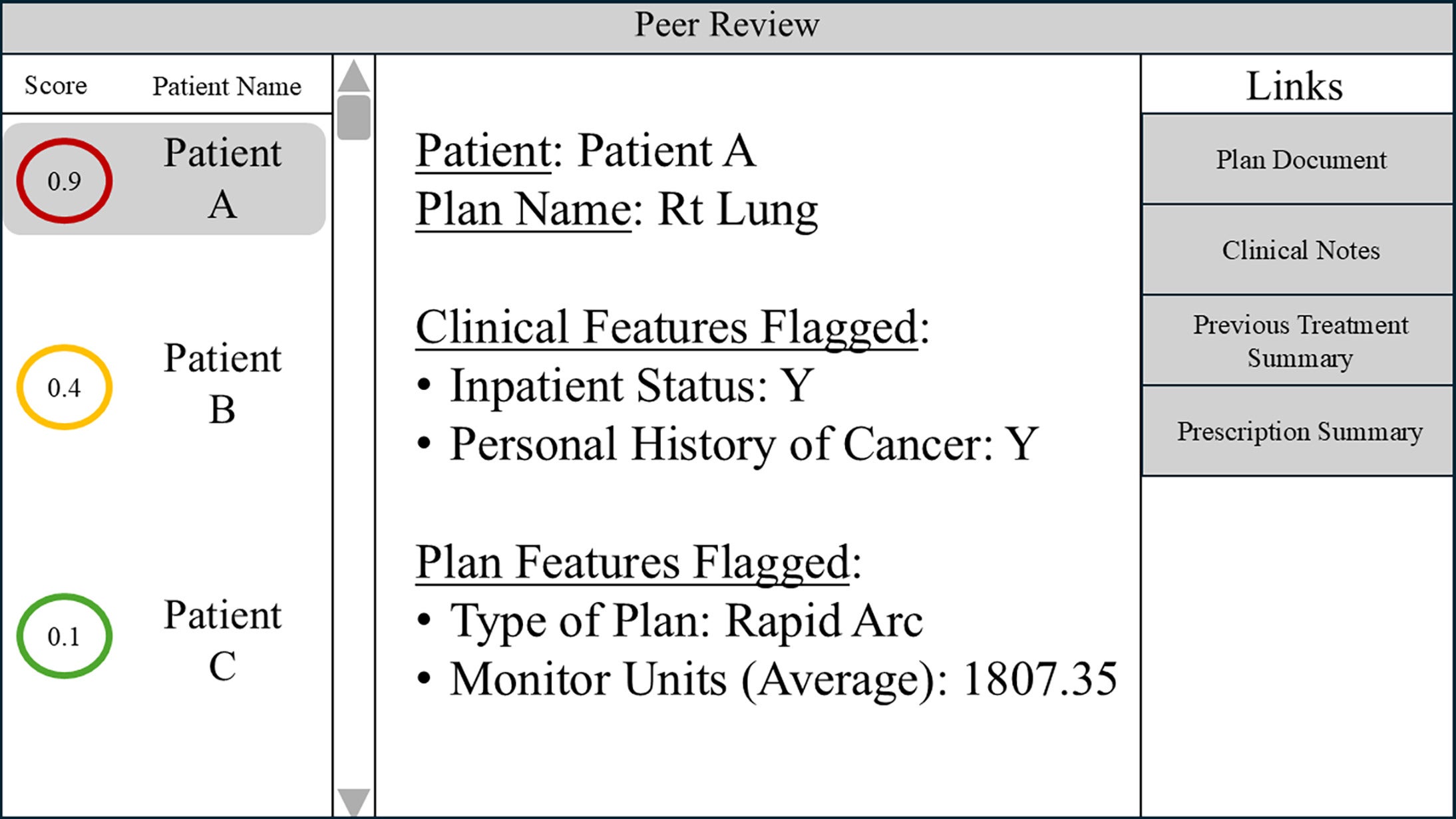
Our study demonstrates the potential of AI in radiation oncology peer review to prospectively identify treatment interruption. A simple example of one potential software display in a peer-review setting is shown in Figure 3 . Each patient could be assigned a “score” associated with treatment interruption risk that could help prioritize cases for discussion. The software might also display the clinical or plan features flagged for that specific case. Links directly to the plan and other relevant clinical documents could facilitate quick reference during discussions.
An example of an application interface that can be displayed during peer review. The application incorporates the risk of treatment interruption score, highlighting features flagged for review and quick access links to patient data.
In radiation oncology, every patient receives a personalized plan chosen by their physician that best fits their characteristics, such as their diagnosis and performance status. However, for reasons that are sometimes unknown, a patient may require a change or break in treatment, which can be detrimental to their oncologic outcome. In patients with head-and-neck cancer, the hazard rate of death increased 4.2% for each additional day needed to finish radiation therapy beyond 8 weeks.13 Even small disruptions in radiation therapy can have negative consequences in gynecologic patients. Lanciano et al reported a 7.7% reduction in 4-year survival when the radiation therapy course was >10 weeks compared with 8.0-9.9 weeks.7
The outcome analyzed in our study was based on whether or not a plan had remaining fractions. Although remaining fractions are not always indicative of treatment toxicity, they could be a major contributor. Several studies have looked at the ability of ML models to predict toxicity using clinical and dose factors and/or radiomic features.14, 15 Reddy et al used random forest, gradient-boosted decision tree, and logistic regression models with input of clinical and treatment variables to predict breast cancer treatment toxicity and achieved an area under the curve (AUC) ranging from 0.56 to 0.85.16 Das et al created a fusion of different non-linear multivariate models (decision trees, NNs, support vector machines, and self-organizing maps) with input of dose and non-dose patient variables to predict radiation-induced pneumonitis in lung cancer patients with an AUC of 0.79.17
The test set accuracy in our study ranged from 57% to 71% for the lung/breast/chest subset. We grouped lung and breast into the same subset due to data size limitations; thus, the predictive accuracy of our models may be improved by further separation.
Similar to their study on breast cancer patients, Reddy et al used random forest, gradient-boosted decision tree, and logistic regression models with clinical and treatment parameters to predict head-and-neck cancer treatment toxicity and achieved an AUC of 0.64-0.76 in their validation set.18 Jiang et al used three supervised learning methods (ridge logistic regression, lasso logistic regression, and random forest) to predict xerostomia, resulting in an AUC of 0.7.19
Our head-and-neck subset came in lower at 53%-61% test set accuracy. We did not directly analyze toxicity because we predicted whether there were remaining fractions in each plan. Remaining fractions could also be caused by re-planning owing to tumor shrinkage; this may explain the reduced predictive value of our study compared to others looking solely at toxicity. Carrara et al applied the artificial NN approach with five input variables relating to patient dose, history, and therapy to predict toxicity after high-dose prostate cancer radiation therapy, achieving an AUC of 0.78.20 Pella et al used support vector machines and neural network-based algorithms to predict acute toxicity of the bladder and rectum due to prostate irradiation, resulting in overall accuracy similar in both models at an AUC of 0.7.21 The accuracy of our testing set in the prostate/pelvis subset, at 71%-75%, is similar.
Although toxicity is one cause of treatment interruption, physician input/suggestions can also cause interruptions if the case is not presented before treatment and/or some information is not assessed during peer review. The traditional approach to peer review is based on “chart rounds,” where the physicians, physicists, dosimetrists, and therapists review details of each case (eg, clinical history, treatment technique, prescription dose, treatment plan, and patient setup). The average amount of time spent on each patient during peer review was reported to range between 1 and 4 minutes.2, 22
Therefore, reviewing the more technical aspects of treatment delivery (eg, monitor units and couch/collimator/gantry parameters), which may provide additional information, may not be feasible owing to time constraints. As demonstrated by our study, subtle, complex relationships within/between clinical data and plan parameters may influence successful treatment delivery. AI and ML have the potential to identify and analyze these relationships for peer review.23 Similar to the goals of many AI-driven studies, ours is not to replace humans with AI but instead to offer providers additional tools to enhance the process. These can supplement clinical intuition and deepen our understanding of factors that previously might go unanticipated.
Since time is a limited resource, AI/ML can also help to identify and prioritize cases that require more time for discussion. Ultimately, these technologies could improve patient safety and treatment outcomes.
Our study offers a concept that can be used to better identify charts requiring more in-depth review. Many software tools for peer review or chart checking take “rule-based” approaches, meaning that the parameter being flagged will need to be predefined with a range or value.24 Azmandian et al used clustering techniques for outlier detection based on treatment parameters in four-field box prostate plans; this study helped to detect plan abnormalities without using the rule-based approach.25 Kalet et al developed a Bayesian network model using clinical and plan parameters to detect errors in radiation therapy plans. Their model utilized a clinical layer (eg, morphology or tumor type), a prescription layer (eg, total dose, dose per fraction, technique), and a treatment layer (eg, monitor unit per fraction, number of beams, beam energy). Their study achieved an AUC of 0.88, 0.98, and 0.89 for the lung, brain, and breast cancer error detection networks, respectively.26 Similar to our study, Kalet et al found the highest testing set accuracy in the brain subset.
Luk et al also used a Bayesian model incorporating prescription, plan, setup, and diagnostic parameters to detect chart review errors. The AUC for this study ranged from 0.82 to 0.89.27 Our testing set accuracy is lower than these other studies, possibly because our cohort included not only “abnormal” cases from a chart-checking perspective but also cases with toxicity-related interruptions.
Although the sensitivity and specificity of our models are relatively low, there are multiple ways in which we can improve these metrics of our study. First, while our study specified remaining fractions as a surrogate for cases likely to experience treatment interruption or complications, remaining fractions can result from reasons unrelated to treatment. Our definition of treatment interruptions as remaining fractions includes patients whose treatments were re-planned and those who discontinued treatment. Separating these cases into two cohorts could improve model predictability.
Second, we grouped subsets based on treatment site, but further dividing them into more focused groups may improve model predictability, eg, separating pelvic plans based on whether they include pelvic lymph nodes or separating spine plans based on vertebral level. Overall, model performance and rigor are expected to improve with increased curation of the dataset and additional clinical factors, including dosimetric plan and structure set data. Additionally, introducing socioeconomic and personal factors, which are commonly seen as causes of treatment interruptions, could improve our models.
Future study directions can also include optimizing hyperparameters and layer structures for the NN. Alternative techniques for data re-balancing or using convolutional NNs for deep learning could also be investigated. A weakness of this study is that it does not include brachytherapy, electron therapy, other treatment sites, or pediatric populations. To address this issue, we are continuing the study with brachytherapy, electrons, and pediatric populations, as well as including additional, more robust parameters.
A known limitation of NNs is their difficulty in identifying the single feature that contributes most to the model, given the complexity of their relationships and the numerous weights/biases assigned to them. Future studies can explore a technique to uncover those features selected by the NN deemed to be most important to the predictive task. At the current stage of our model, which demonstrates moderate predictive performance, making conclusions about or connecting a specific result and/or feature to a reason for a predicted interruption is difficult. Improvements in model performance should enable exploration of more advanced, explainable-AI techniques.
Another current focus of AI and ML is on treatment planning.23 For example, AI is being used to adapt treatment plans in real time to match the day-to-day variations in patient anatomy, thereby reducing interruptions caused by re-simulation and/or discussion manual re-planning.28 As the technology for adaptive planning becomes more widely available, our model will likely need additional training to keep up with technological advancements. Future studies may focus on sites where adaptive planning is often beneficial or necessary, such as the head and neck owing to tumor progression or treatment response. The ability to predict cases requiring adaptive planning due to tumor change can allow the care team to anticipate and minimize treatment breaks.
CONCLUSIONS
Our study demonstrated the ability of AI and ML models to predict major changes in patient treatment, including re-planning and radiation therapy cessation. The findings point to the promising capability of AI and ML to augment peer review and encourage further studies in this aspect of radiation oncology.
References
Citation
Cattell RF, Kim J, Zabrocka E, Qian X, O’Grady B, Butler S, Yoder T, CMD, Mani K, Ashamalla M, Ryu S. Artificial Intelligence-Assisted Peer Review in Radiation Oncology. Appl Radiol. 2025;(3):1 - 11.
doi:10.37549/ARO-D-24-00025
August 28, 2025