CTA of cystic renal masses
Images




Dr. Fielding is an Associate Professor of Radiology and the Chief of Abdominal Imaging, University of North Carolina, Chapel Hill, NC.
At the University of North Carolina (UNC), most cystic renal masses are removed by laparoscopy. Computed tomography (CT) plays a critical role in the success of the procedure by defining renal pathology and creating detailed vascular roadmaps for the surgeon to use in treatment planning.
CT protocol
Table 1 outlines the UNC CT protocol for the staging of renal masses. We do not use oral contrast. We use a 16-slice multidetector scanner, a detector collimation of 0.75 mm, a table speed of 24 mm/sec, a gantry rotation of 0.5 seconds, and a total scan time of 24 seconds. We obtain 3 sets of axial images, each of which is reviewed at 5-mm slice thicknesses at the picture archiving and communications system (PACS) workstation.
We first obtain unenhanced images. Next, we obtain images during the nephrographic phase, 100 seconds after intravenous administration of 100 mL of contrast medium, 350 mgI/mL, at 3 mL/sec through a 20-gauge antecubital line. We then obtain images during the excretory phase, 3 minutes after completion of nephrographic-phase imaging.
We have found that the first set of enhanced images is adequate for review of vascular structures. Not all institutions obtain excretory-phase images, but they can be useful for evaluation of the ureters. If desired, an arterial-phase set of axial images can be obtained with a delay of 25 to 30 seconds and substituted for the excretory-phase images. We then create coronal and sagittal reformatted images, using 5-mm-thick slices reconstructed at 3-mm increments.
To reduce the risk of contrast-induced nephropathy, we require that the serum creatinine level be checked in all hospitalized and emergency room patients within 48 hours prior to the CT scan. Outpatients complete a 6-question form. If the patient has known renal disease, is currently undergoing chemotherapy, or has a long history of treatment with nonsteroidal anti-inflammatory drugs (NSAIDs), we require that the creatinine level be checked. In all other patients, we do the CT scan without determining the creatinine level.
If the creatinine level is >1.8 mg/dL, a radiologist evaluates the patient and considers whether to use isosmolar contrast medium and pre-hydration, 2 methods that have been shown to reduce the risk of contrast-induced nephropathy. 1 In some cases, we may also turn to an alternative imaging method, such as magnetic resonance imaging. At this time, we do not use sodium bicarbonate or acetylcysteine for the prevention of contrast-induced nephropathy.
Bosniak class
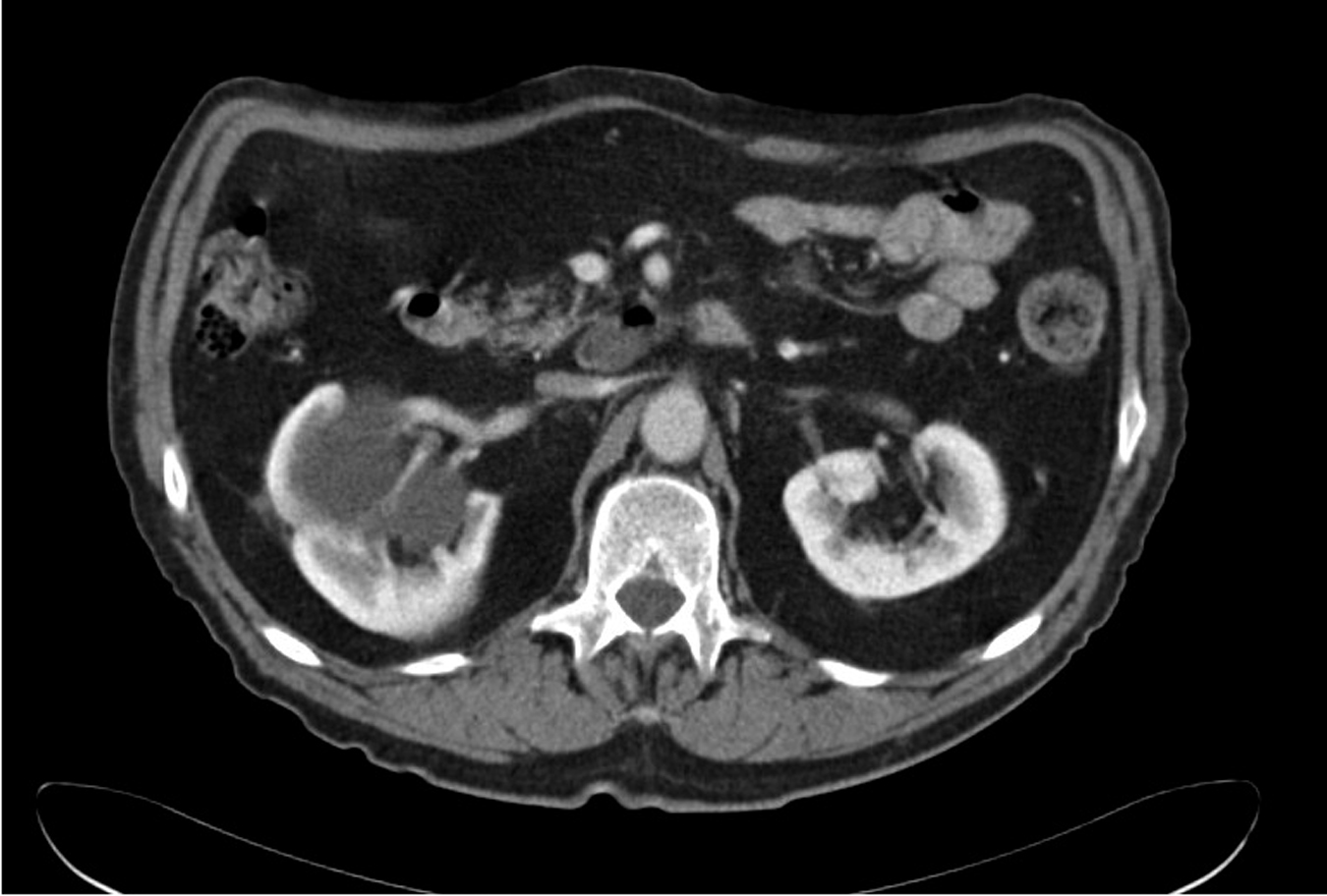
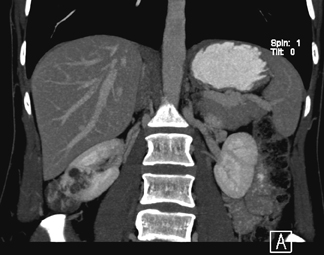
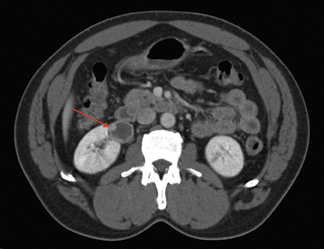
The Bosniak classification system is useful for describing renal masses. 2 Bosniak I lesions are simple cysts with no enhancement, no visible wall, no internal architecture, and clear fluid densities. They are benign lesions, and no follow-up is necessary. Figure 1 shows a simple cyst in a patient with hematuria.
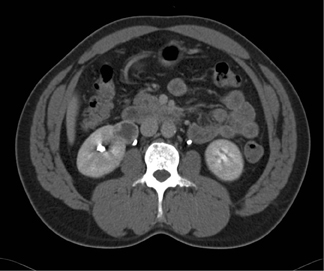
Bosniak II lesions demonstrate no enhancement, a few fine septa, thin rim calcification, and fluid densities (Figure 2). Proteinaceous cysts usually belong in this category. Bosniak II cystic renal masses are usually benign lesions, with a <5% likelihood of malignancy. They usually require no follow-up.
Bosniak IIF lesions do require follow-up. These are small (<1.5 cm), high-density lesions in which densitometry is difficult and the wall cannot be identified. These are usually cortical lesions, usually occur in young patients, and are difficult to diagnose with confidence. Current recommendations call for a follow-up every 6 months for a total of 2 years. Any increase in the size of the lesion indicates a vascular neoplasm in need of ablation or surgical removal, depending on its location.
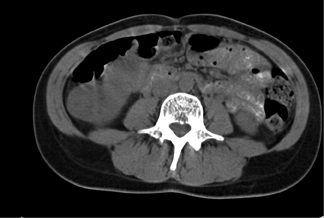
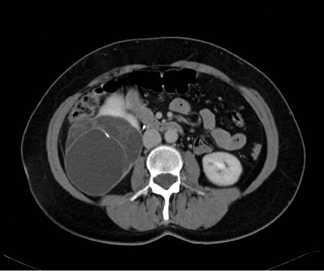
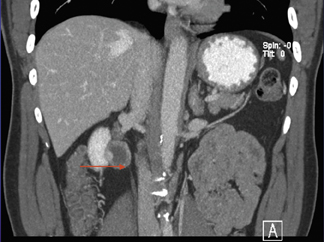
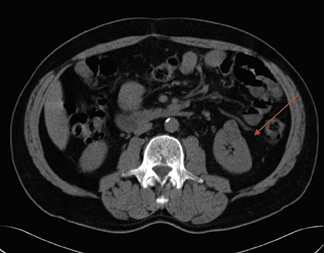
Bosniak III lesions have thick septations, a visible enhancing wall, coarse calcifications, and areas of enhancement >15 HU. Enhancement is the single strongest predictor of outcome, identifying a 25% to 45% likelihood of malignancy. 3 Figure 3 shows a patient with a cystic renal tumor. The mass has >3 thick septa. Intrusion into the renal hilum necessitates total nephrectomy.
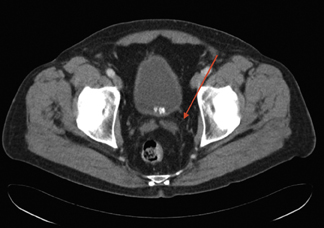
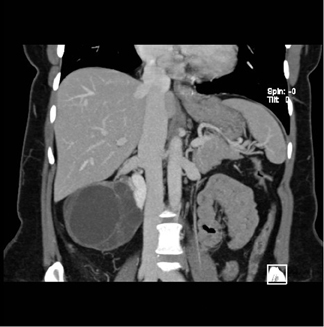
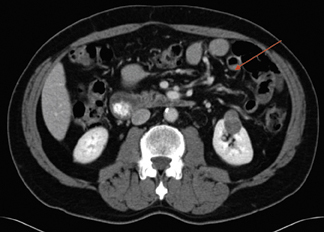
Figure 4 shows a rare case of a multilocular cystic nephroma in a middle-aged woman. There are multiple septa within the lesion, as well as a few calcifications. Delayed imaging illustrates classic herniation into the renal pelvis. Capsular feeder vessels can be seen below the tumor. It is critical to identify these vessels prior to laparoscopy, so that the surgeon can plan trocar insertion and lesion removal accordingly.
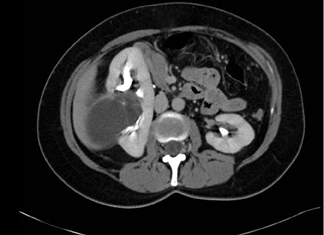
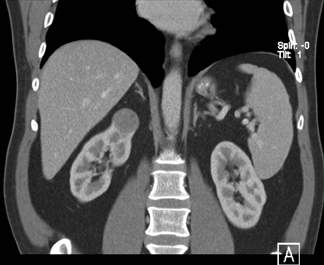
Bosniak IV lesions are cystic masses with a solid wall nodule and enhancement >15 HU. These malignant lesions are almost always renal cell carcinoma (Figure 5).
With advanced CT scanners, we have found it necessary to modify our criteria for identifying malignant lesions. In the past, with a 4- or 8-slice scanner, we used an enhancement cut-off of 10 HU. Be-cause of the pseudoenhancement that occurs with beam hardening, we found it necessary to raise the cutoff to 15 HU with the 16-slice scanner. Some university medical centers use a cutoff of 20 HU.
It is also necessary to be more circumspect when evaluating heterogeneity in the internal architecture of a lesion. This criterion has long been an important aid in the identification of tumors. With 16- and 64-slice scanners, however, there is much less homogeneity in normal tissue, particularly when automatic dose reduction beam modulation programs are used.
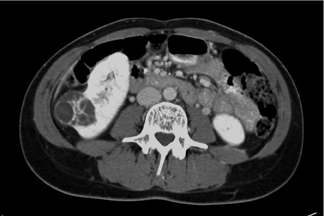
Figure 6 shows a minimally enhancing renal cell carcinoma. Attenuation on the noncontrast image was 44 HU at the upper pole (Figure 6A), only a little higher than the 35- to 38-HU characteristic of normal kidney parenchyma. On the contrast-enhanced, nephrographic-phase scan, attenuation measures 60 HU (Figure 6B). There is some mottling inside the lesion (Figure 6C). The combination of enhancement >15 HU and architectural heterogeneity defines this lesion as minimally enhancing renal cell carcinoma.
Reading the scan
The following information should be included in the radiologist's formal re-port to the surgeon:
• The size, location, and number of renal masses. Partial or heminephrectomy is usually contraindicated for removal of a large polar mass deforming an adjacent calyx, one that abuts the center of the renal hilum.
- The presence or absence of significant enhancement (>15 HU) on nephrographic-phase images obtained using a 100-second scan delay.
- The relationship of the tumor to the renal vasculature and collecting system.
- The presence or absence of enlarged adjacent lymph nodes (>1 cm), and loco- regional or distant metastases.
- The location of capsular feeder vessels above and below the tumor.
Hemorrhage is the most common complication of laparoscopic partial nephrectomy, occurring in approximately 10% of cases. 4 Approximately 25% to 30% of patients have multiple renal arteries. CT can depict accessory renal arteries as small as 2 mm in width. Three-dimensional reformatted images easily show polar arteries, capsular branches, and early hilar branching. 5,6
It is critical that the surgeon also have information on renal vein variants. 7 CT can depict circumaortic, retroaortic, and multiple and early dividing renal veins. It can also reveal the presence or absence of splenorenal shunts, renal vein thrombosis, and intravascular tumor extension. CT aids in the detection of collecting system and ureter anomalies as well. Using excretory-phase imaging, we see bifid renal pelvis in approximately 10% of patients and ureteral duplication in approximately 1%.
Pitfalls
Radiation dose is a concern in CT evaluation of kidney masses. Our 3-scan protocol can expose the patient to a total radiation dose of 10 to 12 cGy. Automatic dose modulation is one possible solution, but it can result in a poor signal-to-noise ratio and questionable HU measurements, thereby undermining the critical role of densitometry in differentiating benign from malignant cystic renal masses. Our solution has been to use an automatic dose modulation protocol with thin patients only. For patients weighing >200 pounds, we select an mA equal to the body weight.
Beam hardening can cause an artificial increase in densitometry measurements, particularly in lesions surrounded by bright cortex. Fortunately, studies have consistently shown that the increase in density is <10 HU. 8 Therefore, most centers now use 15 HU to define vascular enhancement. If enhancement is indeterminate, the best approach is to consider morphology, using the most worrisome feature to guide therapy.
Biopsy
Traditionally, biopsy of renal masses has been uncommon. In the case of solid renal masses, for example, most radiologists believe the probability of renal cell carcinoma is so high that a biopsy seldom influences outcome.
However, two recent studies suggest that renal biopsy of complex cystic renal masses may be useful in identifying patients with benign masses who can safely avoid surgery. 9,10 Some centers are now performing biopsies of renal lesions, but in my opinion, this approach has not yet been adequately studied. Of primary concern is the possibility that biopsy may miss malignant tissue within a cystic lesion.
Renal biopsy is indicated in certain cases. In a patient with known lymphoma or lung cancer, a renal mass represents a primary tumor in approximately half of cases and metastases in the other half. To make this distinction and determine the best course of treatment, it is necessary to do a renal biopsy.
Renal biopsy is also indicated in patients with superimposed renal disease. For example, polycystic kidney disease with a tortuous residual cortex can mimic a renal mass. Similarly, in a patient with a superimposed chronic infection, a biopsy is sometimes necessary to confirm or rule out the presence of a renal mass.
Finally, in a patient with a solitary kidney, it is appropriate to biopsy any mass. Nephrectomy will necessitate dialysis, and a biopsy can assure both the surgeon and the patient that surgery is the right course to take.
Conclusion
With the routine use of laparoscopic approaches for both partial and total nephrectomies, radiologists must provide a complete data set for surgical planning. This includes more than the routine size and location of the mass. A radiologic report now delineates the arterial and venous drainage patterns of the tumor, including the presence of capsular vessels. With respect to cystic renal masses, careful application of the Bosniak criteria, with special attention to lesion morphology and enhancement on multidetector scanners, should lead to excellent results.
Discussion
ELLIOT K. FISHMAN, MD: Thanks very much, Julia. Let me start off the questions. If someone presents with hematuria, what's your basic protocol?
JULIA R. FIELDING, MD: Painful hematuria is a renal stone protocol. Painless hematuria goes to a CT urogram. A CT urogram is done instead of an IVP in the majority of patients now, and is basically a two-run series where we do a 75-second delay, and then we do a 5-minute run. I happen to use a compression device on my patients, rather than use a diuretic or something like that. But it is important to get the urine out into the collecting system and ureters. I have very good results with that.
FISHMAN: What kind of contrast protocols are you using for these studies?
FIELDING: I use 100 mL of 350 mgI/mL contrast injected at about 3 mL/sec most of the time. We have a 16-slice scanner. I use a 0.75-mm collimation for the CT urogram. So it's a hefty dose again. I'm trying to keep it down to a dull roar, especially for the reconstructions.
FISHMAN: In terms of looking beyond the axial plane, is that just for problem solving, or is that something you do routinely?
FIELDING: We do it for every single case. Every single case of painless hematuria, characterized renal mass, and surgical planning for renal mass is done with 3D imaging. So it is pretty routine for us now.
KAREN M. HORTON, MD: So you don't do noncontrast CT? Or do you think you can pick up the stones on the 75-second delay scan?
FIELDING: No, because what I'm doing is I'm trying to separate the patients into 2 groups, basically. It's true that you can have hematuria from stones that are just rattling around, I guess, inside your inter-renal collecting systems. But I have elected to avoid a third run right now. So it may be that I miss small stones, but I'm hoping that I get all the cancers.
FISHMAN: Do you have any difficulty with Bosniak II lesions or high-density renal cysts simulating a tumor?
FIELDING: Sure. Everybody runs into difficulty with that.
FISHMAN: But, if you have a noncontrast CT, the main reason to me is that you can always see the stones. Are the small stones important?
FIELDING: Okay, but remember, though: What bleeds? Not renal cells. Renal cell bleeds rarely. Transitional cell bleeds all the time. So I've actually tailored the two protocols separately, a renal cell protocol for a renal mass. A cortical mass is a different protocol that does include a pre-contrast study. But, to me, an urothelial issue doesn't require one.
FISHMAN: I don't disagree. Just statistically, the amount of TCCs we see compared with renal cell carcinoma is very small. But when a patient's referred to you from the urologist and comes down with hematuria, where it's basically "find something." That's kind of what we're typically looking for.
FIELDING: Right. I had to give them a check-box form that I sent up to them. The check-box form had six boxes on it. It says "painful hematuria. I think this person has a stone-check." Or, "painless hematuria. Assess for urothelial lesion." Or, "preoperative planning for renal cell carcinoma." So they ended up getting a sort of drop-down menu that they use to send things to us.
There certainly are cases where I have someone come in with plain old hematuria, but so far, I guess because I have good help working in a resident-located place, we figure out what's going on from our computer records and decide which test we think is best. So I'm not for one-protocol-fits-all renal cases. Partly that's because I'm a GU radiologist, so I guess I'm more of a splitter than a lumper for that kind of stuff.
GEOFFREY D. RUBIN, MD: I think that was a nice comprehensive overview of renal mass assessment. How frequently are your surgeons doing the nephron-sparing surgery laparoscopically and requiring you to work out some of the smaller venous anatomy? You mentioned the capsular vessels and such?
FIELDING: As long as I can give them the capsular vessels and whether there's an early or a large draining vein, that seems to be okay. The obvious big renal venous anomalies, I think, are okay. So I'm not required to find smaller items than that.
RUBIN: Do you do those studies biphasically to look for venous and arterial anatomy? Or you try to get them in one phase?
FIELDING: I get them at one time. That's what I'm doing right now, which I know is a little unusual.
RUBIN: So what's your delay and how long of an injection are you giving?
FIELDING: I give a bolus of 100 mL at about 3 mL/sec, and I use a 100-second delay.
RUBIN: And you still have arterial opacification?
FIELDING: I do. I know it's a weird protocol, but to me, it's so critically important that I get the correct enhancement on those things, which is why I've chosen that. But you could certainly do an arterial-phase image first.
RUBIN: I guess if it was important for my surgeons to know whether there was a tiny accessory or capsular artery, I would be concerned that waiting 100 seconds with effectively a 30-second bolus would leave me with insufficiently opacified arteries. What do you do?
FISHMAN: We do a lot of partial nephrectomies. That's one of the most common indications we have for doing CTs. We will do it with arterial-phase imaging, because they demand really detailed arterial maps from us. We do the arterial, venous, and we might get a delayed image. To evaluate a big renal mass, just to stage the tumor, I don't worry about noncontrast scans.
But the other thing you can do just for noncontrast scans through the kidneys is 5-mm scans; I always can see stones on arterial-phase imaging. If the stones are there, you see them anyway. So the only value to me of the noncontrast CT, quite frankly (besides knowing the kidneys are where they're supposed to be) is determining lesion density. So if I had a high-density renal cyst, I could look sequentially from non-contrast through delayed images.
Particularly in this era of 64-slice scanners, we can get fantastic angiographic maps that I think really make the difference in whether or not they can do partial nephrectomies in borderline cases. The partial nephrectomies are also done routinely laparoscopically, which makes a significant difference. If you go by strict criteria, previously it was 4 cm, exophytic, and peripheral--those are the simple cases. If you find those, it's not a major to-do. But the surgeons are getting more and more aggressive, particularly as we're picking up patients in their 50s with renal masses. So our experience has been that they want that level of detail. Obviously, you want to make sure that the patients you're selecting for partial nephrectomies will end up with good five-year results. So we will do that arterial-phase image.
I can see what Geoff was alluding to, that with the 100-second delay, I can see the renal arteries, but I just don't have the detail. Also, in terms of venous mapping, if I'm doing really good venous mapping, a 70-second delay tends to be good. We're typically using 100 mL of contrast at 4 mL/sec on a 16- or 64-slice scanner. That gives a really good venous opacification. I've not made any mistakes in terms of flow-related change in either the IVC or the renal vein. When the tumor does extend beyond renal vein into IVC, we've been very good at being able to determine exactly how far the IVC goes, whether it's above the diaphragm or not.
As you said, the postprocessing is critical. We do a combination, a little like what you showed, 3D mapping using volume rendering and MIP, and then some coronal displays, which tend to work fairly well. We do use sagittal images occasionally, particularly if we're uncertain if a lesion is renal, adrenal, or liver. But I tend to be one who likes that early-phase imaging.
RUBIN: Particularly recently, with the 16-row and faster scanners, if you trigger right on the arterial enhancement, you can routinely see interlobar arteries of the kidney. Then it becomes a question as to whether the surgeons are going to use that information in planning their surgery, and it becomes a bit of an "if-you-build-it-they-will-come" phenomenon. If they've never known that you can show it to them, then they might say, "Oh, what you're giving is just fine." But if you can show them intrarenal branches and their relationship to the tumor, they might say, "Gosh, I'd like that in every case." Are you alluding that the demonstration of intrarenal branches is impacting on the surgeon's decision?
FISHMAN: Yes. I think the big thing now is that surgeons are becoming more aggressive as we give them more detailed mapping. As I said, they used to go after the peripheral exophytic lesions. Now they going for more of the central ones, where they do much finer dissection than they did before. I think the other issue is that you might have a problem with the venous invasion. But if you're just looking in terms of defining venous anatomy, typically at 30 or 40 seconds, you have all the venous anatomy anyway. So the only time the venous phase per se helps is if you have IVC extension, and maybe at 40 seconds you have some flow-related changes. But in terms of defining venous anatomy, Satomi Kawamoto at Hopkins actually wrote an article in AJR that said that basically from the 30 seconds you get all the arterial and venous images; and that's based on looking at renal donors.
RUBIN: Are you doing renal donors uniphasic now?
FISHMAN: We are starting to, yes. Now it's interesting, as Geoff said, in some ways, what you provide the referring docs is what they expect you to provide. So on all of our hematuria cases and in all our renal cases, we routinely do CT urography. Since you need delayed-phase imaging anyway and delayed phase will give you that differential enhancement. We always reconstruct with CT urography routinely, in every patient. So, if you start looking at numbers, for your arterial-phase image, 85% of renal cells enhance. So right away, you can have some really high numbers. So, when looking at a large number of cases, the reality is that if you have arterial-phase and excretory-phase images, you always see differential enhancement.
Now the times it may not happen are small. If you have a relatively hypovascular renal cell, it may not be a substantial difference between the early-phase and late-phase imaging; but otherwise, it does work pretty well. I think the issue is also that if it weren't a radiation issue, you'd get four phases in everybody; that would be very simple.
So the question is, how do you work around minimizing the number of acquisitions while maximizing the information?
ELLA KAZEROONI, MD: How much of the postprocessing is physician-driven? Is it an entirely physician-driven process for renal mass?
FIELDING: It's done by technologists for the pancreas, for the liver, for the kidneys--basically, the visceral organs. Those have standard technological processing that's done by the technologist. Then we have a workstation in our room that we can do extra stuff on. But I don't do it as often as you do. I would say that maybe 20% of the time I want to look at it some other way and I cut it some other way.
RUBIN: I think that's true in our practice for solid organ work. Our renal postprocessing protocol is very specific from the 3D lab and it's usually sufficient. We get thin-slab MIPs along the axis through the axis of the kidneys, and then we get the whole kidney views. We also measure cortical and whole renal volumes on every patient. That's something that our urologists and nephrologists are demanding. They really like that, and it's a very quick segmentation of cortical volumes.
FIELDING: For most of the visceral stuff, it is actually pretty easy to just set and go.
FISHMAN: Well, I'll disagree 100%! We do them all ourselves.
FIELDING: But is that not a waste of physicians' time?
FISHMAN: No. I think we have the best technologists at Hopkins, or as good as anybody. They know how to do 3D. But the bottom line is the way you look at a kidney is in 3D. You can look at the combination of axials, coronals, and sagittals. The only reasons you do it the way you do it now are tradition or the lack of available computer resources.
FIELDING: It's also use of physician time.
FISHMAN: Not to be argumentative, but I guarantee you that I could do a 3D faster than you could look at axial images.
FIELDING: But you are an isolated case.
FISHMAN: No, all you've got to do is put the time in.
KAZEROONI: Do you save the 3D images for the physicians on the PACS? Or do you only process them for your own use?
FISHMAN: We're doing about 150 CTAs a week, or more. When I look at them, I film the key images as I'm looking--these key images are between 20 and 30 images, depending on the case. They're printed out like high-res film and they're delivered to the physicians at two o'clock every day.
KAZEROONI: What about when you go filmless?
FISHMAN: The thing is, this has nothing to do with being filmless. You can have filmless radiology; you can have everything on the Web. But physicians like to have images in their hands. Now we don't give them 14″×17″film; the film is 8″×11″. I can tell you that pancreatic surgeons and renal surgeons take those images, they look at them, and they show them to their colleagues. They put them in the patient's report so they can bring them up and take them to surgery. So, although you're filmless, the cost for cameras is not much, less than $500. You plug them on your workstation.
FIELDING: Do you take the pictures, too?
FISHMAN: Yes, because you do it when you're looking and no one knows better than you. If you tell me that technologists know as much as you do, then we have a problem with reimbursement. Then you're being overpaid!
FIELDING: But you're being underpaid if you're wasting your time doing all this.
FISHMAN: No, I'm not being underpaid. That's why you have 2 hands and 10 fingers. You do everything at one time--multitasking. So as far as I'm concerned, you look, you film, you talk, it's over. Boom. That's it. I don't have to go get the films to look at what someone else did. I do it at one time. It's over.
KAZEROONI: When I bring up a CT, I'd like to see the images at least in the coronal, axial, and sagittal planes, and be able to scroll through, thicken or thin them up as I need to, and adjust window settings, interactively. For specialized protocols, like coronary CT angiography, I spend much more time using the advanced processing workstations. The place-holding function or "file room" function of having some standard proto-col-driven images is done by our 3D lab techs. I can then do additional processing on the cases I feel it's needed on. Then, as far as film, I can't imagine giving film to one of my surgeons anymore. It's all filmless. Or, I can send images to our server and put them quickly into PowerPoint so I can e-mail them, and their response to the latter is always very positive and I'm met with a lot of gratitude.
FISHMAN: We do that, too. We save interesting images. But it's an interesting philosophy. What volume of CTAs are you doing in a week at Michigan?
KAZEROONI: We're divided up into organ specialties. Just in thoracic radiology alone we're probably roughly doing 50 to 60 a week. But we have thoracic, abdominal, and neuroradiology divisions all doing CTA.
FISHMAN: One of the simple things that we find is that with our pancreatic surgeons and most of our GI surgeons, not only do they want to see the images, they want to see them in real-time. So even sending them images, that's cool. They want to physically come down. John Cameron, who is professor of surgery and ex-chair of surgery, who has done more Whipple procedures than anybody in the world, will routinely not do a Whipple without seeing the study in 3D. You know, he wants static images, but he wants you to be roaming through the data sets.
So one of the things you're seeing changing is what the referring physicians expect to get from us. For years, referring physicians got films of a few images, or they got a few things on the PACS. That's not good enough. Our guys don't want to see axial images, and they don't even want to see a few 3D images. They want the interactivity. That's where I really see things going, because as workstations get faster, as you get the load times, the interactivity increases. That's why 64-slice CT is a disruptive technology. If you're giving axial images or just a few simple things, it really minimizes what you get out of the data set.
KAZEROONI: Technology is wonderful, right? Whether it's sending a few specialized coronal MIPS or whatever it is you want to do, you could send a coronal stack of 30 images in a PowerPoint file and they could open it up whether they're at home or in their office or in the surgical suite. They can have the semblance of interactivity by scrolling through it in a PowerPoint file, similar to what they could appreciate on a PACS workstation.
FISHMAN: But what I think is that you tend to underestimate your expertise. The thing that you get paid for as a radiologist is your expertise. As Geoff was showing those images, it's choosing the right image that tells the answer. You could have 100 images that don't tell the story as well as the 1 right image. That's what I get paid for, that 1 image, not the other 97 images. So I think it's an evolving process. We've been evolving for 20 years and I said the same thing in 1986. So it's just getting closer to the truth now!
It also will impact on reimbursement. The reimbursements for 3D will be different. If a technologist is generating these coronal and sagittals, you get zero reimbursement now. That's gone. So unless you're doing postprocessing yourself, you will not get reimbursed. That's a very practical aspect of reality--if you do it, you will get paid physician time.
RUBIN: I do think that it's important to dispel the notion that all technologists are capable of doing is pumping out coronals and sagittals. They certainly can be trained to do more.
Techniques for lower-extremity CT angiography