ReCODE Protocol Improves Cognition for Early-Stage Alzheimer's Patients
 Early results of a clinical trial validate Apollo Health's ReCODE Protocol, an intervention designed to reverse cognitive decline. After hundreds of failed Alzheimer's trials, the ReCODE protocol represents a giant leap forward in treatment.
Early results of a clinical trial validate Apollo Health's ReCODE Protocol, an intervention designed to reverse cognitive decline. After hundreds of failed Alzheimer's trials, the ReCODE protocol represents a giant leap forward in treatment.
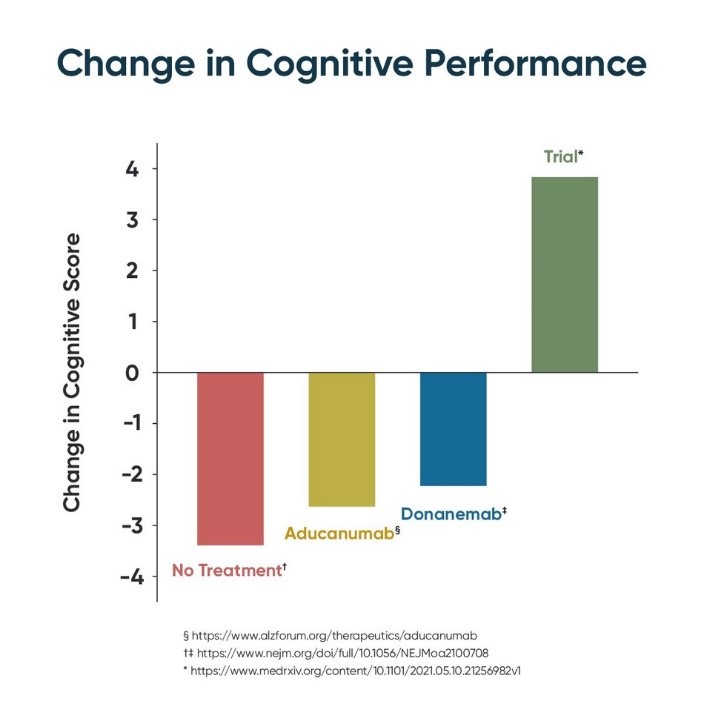
The validation comes from a trial based upon the science of Dale Bredesen, MD that was recently shared in a pre-print entitled Precision Medicine Approach to Alzheimer's Disease: Successful Proof-of-Concept Trial, posted in medRxiv, the Yale-backed pre-print server for health sciences. The trial utilized the ReCODE Protocol and showed the first-ever improvement in cognition in early-stage Alzheimer's patients.
In the team's study, 84% of subjects experienced significant improvement over multiple metrics of cognition including MoCA, CNS Vital Signs, Brain HQ, AQ-21, and AQ-C.
Instead of pre-determining a single-pharmaceutical treatment, ReCODE identifies and targets root cause contributors to cognitive decline for each person. These assessed potential contributors include inflammation, insulin resistance, nutrient and hormonal deficiencies, specific pathogens, toxicants, and biotoxins, as well as genetics. This process has led to unprecedented results. In addition to 84% of patients experiencing a cognitive improvement, there were reported improvements in symptoms and MRI gray matter volumes as well.
While most scientific breakthroughs take decades to hit the market, ReCODE is poised to help people quickly, as it is already available for consumers via Apollo Health. There are trained practitioners in almost every state and in many international locations.
Related Articles
Citation
. ReCODE Protocol Improves Cognition for Early-Stage Alzheimer's Patients. Appl Radiol.
July 1, 2021