Utility of 64-slice CT to monitor changes in atheroma volume: A pilot study
Images



Dr. Ibanez is a Cardiologist and is part of a multidisciplinary research team with a special focus in Cardiovascular Imaging at Mount Sinai School of Medicine, New York, NY. He received his MD in 1993 from The Universidad Complutense de Madrid in Madrid, Spain. He performed his Residency and Fellowship in Cardiology at Fundacion Jimenez Diaz, Madrid, Spain. He received several honors and awards from the Spanish Society of Cardiology. He has published a number of articles and has received Young Investigator award nominations at the European Society of Cardiology Annual meeting in 2007 and the North American Society for Cardiac Imaging in 2005.
The assessment of changes in atherosclerotic plaque volume is
increasingly used as a surrogate endpoint in clinical trials
evaluating the effects of antiatherogenic therapies. Currently,
intravascular ultrasound, cardiovascular magnetic resonance, and
carotid intima media thickening are the modalities usually used.
The utility of multidetector computed tomography (MDCT) for this
purpose has not yet been assessed. This article presents the
results of a pilot study in rabbits to evaluate the use of MDCT
to monitor changes in atheroma volume.
Methods:
Abdominal-aorta atherosclerotic lesions were induced in rabbits
(n = 7), which were then randomized to 2 doses of placebo or a
drug with known plaque-regressing properties (apoA-IMilano). Pre-
and posttreatment 64-slice CT angiographic studies of the aorta
were performed.
Results:
Plaque burden by MDCT regressed by 29% in apoA-IMilano-treated
animals while it did not significantly change in the placebo
group. In the last MDCT study, plaque volume was 10.6% smaller in
apoA-IMilano animals, which corresponded to the postmortem
histologic analysis (11.5% smaller plaque in the apoA-IMilano
group).
Conclusion:
Multidetector CT is a promising tool to assess changes in
atheroma burden.
Atherothrombosis is the major cause of morbidity and mortality worldwide. Atherosclerosis is characterized by lipid deposition (along with other inflammatory material) within the arterial wall, leading to plaque formation. At the early stages of atherogenesis, the lumen of the vessel is not affected because of the "positive remodeling" of the external elastic membrane and eccentric plaque growth; therefore, the luminogram of the atherosclerotic vessel may appear to be "normal." Hence, imaging modalities that are capable of accurately visualizing the entire vessel wall have gained enormous interest in order to quantify the atherosclerotic burden.
Until recently, atherogenesis was envisioned as a progressive cumulative phenomenon of lipid deposition; however, today it is well known that lipid-lowering interventions are able to halt progression 1,2 or even to regress atheroma. 3 Although there is no data correlating atheroma regression with a reduction in clinical events, there is a large body of indirect evidence highly suggesting this association. 4,5 If this hypothesis is confirmed definitely, future clinical trials will use imaging-based studies as a surrogate for clinical endpoints. Current imaging modalities that are used to monitor changes in atheroma burden include intravascular ultrasound (IVUS), 1,3,6 cardiovascular magnetic resonance (CMR), 2 and vessel wall intima media thickening (IMT) by surface ultrasound. 7
Multidetector computed tomography (MDCT) is an emerging noninvasive imaging modality that combines high spatial and temporal resolution with a very short scan time. MDCT has recently gained significant attention because of its ability to accurately depict not only arterial calcification and lumen size, but also the presence and morphology of nonstenotic, noncalcified plaques. 8-10 Hence, MDCT may represent an attractive alternative imaging modality to noninvasively track changes in the atheroma burden. However, to date, there are no available data on the utility of MDCT to accurately monitor atherosclerosis progression or regression. We have recently shown, in a CMR-based preclinical study, that the administration of the experimental drug apoA-IMilano (ApoA-IM) induces marked plaque regression in just 4 days, which may offer a useful tool to explore the ability of MDCT to monitor changes in atheroma burden. 11
The aim of this study was to assess whether MDCT is able to detect and quantify the changes in the atheroma burden of the abdominal aorta that are associated with the administration of apoA-IM in an established animal model of atherosclerosis.
Methods
Study design
Abdominal aorta atherosclerotic plaques were induced in rabbits (n = 7) by a combination of 9 months of 0.2% cholesterol-enriched diet and 2 aortic balloon denudations. The atherosclerosis induction protocol is described in detail elsewhere. 12 To replicate the clinical scenario of statin therapy, the cholesterol content of the atherogenic diet was then reduced by 50% immediately before the first administration of apoA-IM or placebo. Afirst (baseline) MDCT aortic angiography study was performed, and animals were then randomized to receive 2 intravenous injections, 4 days apart, of 75 mg/kg of ApoA-IM (n = 4) or an equal volume of placebo (n = 3). Four days after the last dose, a second (final) MDCT angiography was performed. Subsequently, the animals were euthanized and the abdominal aorta was fixed for further morphologic analysis. Serial sections were obtained at 3-mm intervals to match the corresponding MDCT images, as previously described. 13 Specimens were embedded in paraffin, and 5-µm-thick sections were cut and stained with combined Masson's trichrome elastin stain. The detailed protocol is explained elsewhere. 13
The study protocol was approved by an institutional animal research committee, and all animals received humane care in compliance with the "Guide for the Care and Use of Laboratory Animals."
Noninvasive MDCT protocol
Multidetector CT studies were performed with a 64-slice MDCT scanner (Sensation 64, Siemens Medical Solutions, Forchheim, Germany). For the scans, the rabbits were sedated by intramuscular injection of ketamine (30 mg/kg) and xylazine (2.2 mg/kg). An intravenous line was placed in the central vein of each rabbit's ear with a 21-gauge line. Before initiation of the protocol, different dilutions of the nonionic, low-osmolar, iodinated contrast agent ioversol (Optiray 370, Tyco Healthcare/Mallinckrodt, St. Louis, MO) were tested in an animal not included in the study. The purpose was to determine the optimal concentration leading to intra-aortic lumen attenuation similar to that routinely obtained in clinical human coronary CT angiography examinations (250 to 350 HU). The mixture used in this study was a 1:2 dilution of the contrast agent with saline, yielding a concentration of 123.3 mg I/mL. The animals were imaged in the craniocaudal direction and in the supine position. An initial localizer image served to confirm an adequate position of the animal and prescribe the angiographic study covering the abdominal aorta. The contrast dilution was then infused (8 mL at a rate of 0.5 mL/sec), and scanning was initiated after a fixed delay of 10 seconds was used to trigger image acquisition. Imaging parameters were as follows: 120 kV, 180 mA, rotation time 330 msec, 32 × 0.6 mm collimation, and pitch 0.45. The total acquisition time ranged from 6.5 to 7.5 seconds. Axial images (3-mm thickness with no overlap) were reconstructed using a sharp kernel (B46f), a field of view of 160 × 160 mm, and a 512 × 512 matrix, resulting in an in-plane spatial resolution of 400 × 400 µm. 14
MDCT data analysis
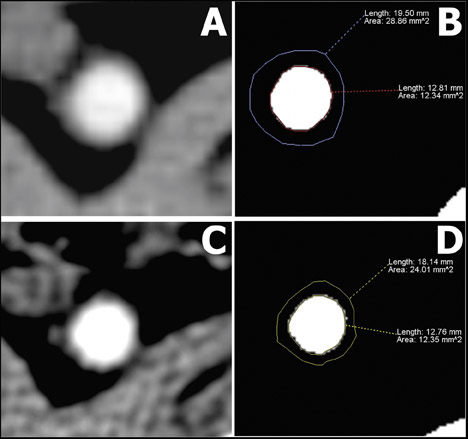
The MDCT images were transferred to a dedicated workstation for analysis. The 17 consecutive 3-mm slices immediately distal to the left renal artery were prespecified as the area of study for the pre- and posttreatment MDCT studies. Because plaque visualization is affected by the attenuation of the lumen, 15,16 a region of interest (2 to 2.5 cm 2 ) was placed in the center of the vessel in each of the slices, and the average attenuation was recorded. The mean luminal attenuation in each study was calculated by averaging the values of the 17 individual slices. Afterward, the display settings were manipulated as follows: for lumen quantification, the window level was brought to 65% of the mean luminal attenuation, and the window width was reduced to 1. For detecting outer vessel boundaries, the window level was kept unchanged, and the window width was brought to 155% of the mean luminal attenuation. These settings were chosen because they have been shown to represent the optimal values for plaque-size measurement when compared with coronary IVUS examinations. 8,17,18 This combination generates a "black and white" image for the detection of the lumen area and a discernible outer border of the vessel wall depiction (Figure 1). Each of the 17 axial sections were manually traced by 2 independent researchers who were blinded to the time of the study and to the treatment arm. Plaque volume of the individual segments was calculated as the difference between total vessel volume (3 × vessel area) and lumen volume (3 × lumen area).
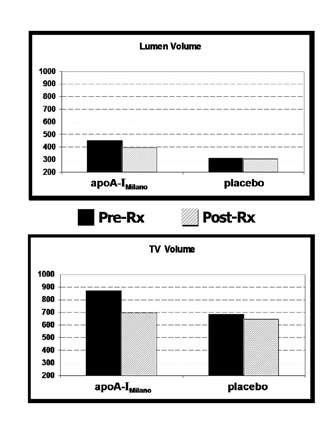
The primary endpoint of the study was the change in plaque burden be-tween the initial and final MDCT studies. Plaque burden was quantified as the total plaque volume, calculated by adding the plaque volumes of each of the individual slices within the aortic segment of interest. In addition, total lumen volume (the sum of individual slice lumen volumes) and total vessel volume (the sum of individual slice vessel volumes) were computed.
A secondary analysis included the change in the individual (slice) plaque volumes. For this comparison, pre- and posttreatment images were matched by its distance from the left renal artery.
Histopathologic analysis
An observer who was blinded to the treatment arm performed the immunohistochemical analysis using a computer-based validated semiautomatic quanti-fication method programmed on ImageJ (National Institutes of Health, Bethesda, MD) and determined the plaque area (vessel area-lumen area).
Statistical analysis
Continuous variables are expressed as the mean ± standard error of the mean (SEM). Statistical comparisons of means were made by Wilcoxon Signed Rank test. A value of P <0.05 (2-tailed) was considered statistically significant. All statistical analyses were performed with the statistical software package SPSS 11.0 (SPSS Inc., Chicago, IL).
Results
Contrast-enhanced 64-slice MDCT angiography was performed successfully in all animals, resulting in adequate image quality for analysis. Figure 2 shows the results of the primary analysis of the study. A statistical significant plaque burden regression (-29%, P = 0.031) was observed in the apoA-IM group between pre- and posttreatment MDCT studies, while no significant change was observed in the placebo animals.
A nonsignificant reduction in total lumen volume was observed in the apoA-IM group, while no effect was found in the placebo group (Figure 3A). A change in the total vessel volume showed a strong trend toward external elastic membrane shrinkage in the apoA-IM group (20% regression versus pretreatment MDCT, P = 0.1), whereas no change was documented in the placebo group (Figure 3B). Figure 4 illustrates individual changes in total plaque burden.
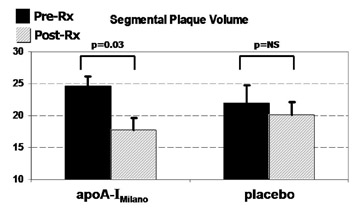
At the slice level, changes in plaque size followed a comparable pattern. The mean segmental plaque volume regressed in the apoA-IM group (-28% versus pretreatment MDCT, P = 0.03), whereas no change was observed in the placebo group (Figure 5). Overall, there was good agreement between the final MSCT study and the histological analysis. In the posttreatment MDCT, the plaque burden of the apoA-IM-treated animals was 11.5% smaller compared with that of the animals that received placebo, although this comparison did not reach statistical significance. At the slice level, the mean plaque volume of the apoA-IM animals was 11.5% smaller than that of placebo animals ( P = 0.018). In the histologic analysis, the mean plaque area was 10.6% smaller in the apoA-IM group ( P = 0.005).
Discussion
In the current study, the hypothesis that MDCT is able to assess changes in atheroma burden has been tested. Abdominal aorta atherosclerotic lesions were induced in a well-established research model of atherosclerosis. 13 All animals underwent 2 imaging studies, and, thus, every animal served as its own control. Plaque burden was significantly reduced in animals receiving apoA-IM, while nonsignificant changes were seen in the placebo animals.
Change in plaque volume is currently one of the major surrogate endpoints used in clinical trials. The advent of new imaging modalities made it possible to accurately monitor changes in plaque volume, while before only the vessel lumen was quantifiable. Such imaging modalities include IVUS, CMR, and carotid IMT. All these imaging modalities have certain issues that limit their global acceptance. Intravascular ultrasound can accurately depict coronary atheroma, and it has been shown to reliably monitor changes in atheroma burden; however, its invasive nature makes its use limited. Cardiovascular MR is an extremely accurate tool to quantify and monitor changes in atheroma burden, but currently the coronary territory is not well depicted because of its limited temporal resolution. Carotid IMT is a noninvasive, safe, and reproducible imaging modality that can precisely examine changes in wall thickness. However, its usage is limited to the carotid region, and the type of lesions developed in that area are widely different from those in the coronary and aortic arteries. Multidetector CT is a noninvasive new modality that potentially can overtake all limitations stated above. In fact, few studies have reported the accuracy of MDCT in quantifying the atherosclerotic plaque burden. Those studies validated the MDCT analysis with well-established imaging modalities: IVUS or CMR. 10,13,17 However, so far, there is no data on the value of MDCT to monitor changes in atheroma burden. To the best of our knowledge, this is the first report showing serial imaging studies for the quantification of the atheroma burden.
Currently, there are several lipid-altering interventions that have been proven to regress plaque burden; however, the time needed to achieve this effect is very long. 1,3,19 ApoA-IM is a mutant form of apoA-I that has been shown to significantly regress atheroma volume in all the animal studies so far reported. 20-22 It has also been tested in humans, showing a significant coronary plaque regression in just 5 weeks. 23 We have recently reported, using the same atherosclerosis model as described here, that only 2 infusions of apoA-IM in 4 days induced a significant reduction in abdominal aorta plaque volume, as assessed by CMR. 11 Due to its unequivocally known rapid plaque regressing effects, apoA-IM represents the ideal tool to explore the utility of MDCT to monitor changes in plaque burden. Usually, the effect of a new intervention is assessed with an established imaging modality; here, we have done it the other way around: using a drug with known plaque-regressing effects, we have tested the feasibility of using MDCT to monitor changes in plaque burden.
Besides the analysis of coronary stenosis and calcification, nonstenotic plaque quantification by MDCT is an emerging area of interest both in the research and the clinical arenas. Early studies heated the enthusiasm on the ability of MDCT to quantify and characterize atherosclerotic plaques. 9,24-26 Several studies have correlated the plaque measurements with that of IVUS. Achenbach et al 10 reported a sensitivity of 82% and a specificity of 88% of MDCT in detecting any kind of plaque. Those values were higher for calcified than for noncalcified plaques. Also, the same group reported that in selected images with good quality, MDCT can assess the remodeling index of coronary artery lesions, correlating well to IVUS measurements. 27 Therefore, MDCT seems to be a reliable modality to assess the lumen and the external vessel wall boundaries. Leber et al 8 showed that 64-slice CT can accurately depict coronary artery plaque burden, showing a significant moderate correlation with IVUS. However, the interobserver variability for plaque burden quantification has been reported to be high, varying from 17% to 37%, according to the size of the vessel and to the amount of disease (the higher the amount of disease, the lower the variability). 8,28
Since plaque visualization is affected by the attenuation of the lumen, 15,16 we decided to use a display setting that takes this parameter into account. However, at this point we cannot be sure that the vessel wall measurements could have been randomly affected by this parameter.
Conclusion
In an animal model of atherosclerosis, the administration of apoA-IM (a drug with known rapid atheroma-regressing effects) was used to explore MDCT utility to monitor changes in atheroma burden. Atheroma burden in animals receiving apoA-IM was 30% significantly smaller in the posttreatment MDCT study compared with the pretreatment study. Placebo administration resulted in no effect on plaque burden. Posttreatment plaque burden in apoA-IM animals was 10.6% significantly smaller than in those treated with placebo, in agreement with the 11.5% significant smaller plaques found in histology. These results suggest that MDCT may serve as a tool to explore serial changes in plaque volume.
Acknowledgments
The author is indebted to the mentorship from Drs. Juan J. Badimon, Mario J. Garcia, and Valentin Fuster. In addition, the great work by Drs. Javier Sanz and Susanna Prat-González, which was crucial for the proper study design and performance, should be acknowledged. Drs. Carlos G. Santos-Gallego and Juan Benezet-Mazuecos significantly contributed to the performance and analysis of the data. Finally, the author would like to thank Dr. Badimon's laboratory at Mount Sinai School of Medicine for all the help and support.
The drug administered to the animals (apoA-IMilano, ETC-216) was provided by Pfizer Research and Development, USA.